-
Name
1-Methylindole
- EINECS 210-057-5
- CAS No. 603-76-9
- Article Data287
- CAS DataBase
- Density 1.008 g/cm3
- Solubility insoluble in water
- Melting Point 94 - 95oC
- Formula C9H9N
- Boiling Point 239.377 °C at 760 mmHg
- Molecular Weight 131.177
- Flash Point 98.572 °C
- Transport Information
- Appearance deep yellow viscous liquid with a very unpleasant smell
- Safety 22-24/25-37/39-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Indole,1-methyl- (7CI,8CI);1-Methyl-1H-indole;N-Methylindole;NSC212534;
- PSA 4.93000
- LogP 2.17830
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.5h; | 99% |
| Stage #1: indole With sodium hydride In tetrahydrofuran Inert atmosphere; Stage #2: methyl iodide at 0 - 20℃; for 0.5h; Inert atmosphere; | 99% |
| Stage #1: indole With sodium hydride In tetrahydrofuran at 0 - 20℃; for 1.25h; Stage #2: methyl iodide In tetrahydrofuran at 0 - 20℃; for 0.5h; | 99% |

| Conditions | Yield |
|---|---|
| With H8O20P8Pt2(4-)*4C34H72N(1+) In methanol Inert atmosphere; Irradiation; | 99% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride In para-xylene at 130℃; for 20h; Catalytic behavior; Temperature; Solvent; Reagent/catalyst; Inert atmosphere; Sealed tube; | 99% |
| With oxygen In N,N-dimethyl acetamide at 20℃; Schlenk technique; Irradiation; | 95% |
-
-
180623-97-6
2,3-diiodo-1-methyl-1H-indole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-diiodo-1-methyl-1H-indole With tert.-butyl lithium In tetrahydrofuran; pentane at -78℃; for 0.333333h; Stage #2: With ammonium chloride In tetrahydrofuran; water; pentane at -78 - 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone | 99% |
| Multi-step reaction with 3 steps 1.1: n-BuLi; CO2 / tetrahydrofuran / -78 °C 1.2: t-BuLi / tetrahydrofuran / -78 °C 1.3: 91 percent / ICH2CH2I 2.1: 100 percent / KOH / dimethylformamide 3.1: n-BuLi; ZnCl2 3.2: Pd(PPh3)2Cl2; 2-iodoindole View Scheme | |
| Multi-step reaction with 4 steps 1.1: 90 percent 2.1: I2; KOH / dimethylformamide 3.1: n-Bu4NHSO4 / dimethylformamide 4.1: t-BuLi / tetrahydrofuran; pentane / 0.33 h / -78 °C 4.2: 99 percent / NH4Cl / H2O; tetrahydrofuran; pentane / -78 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: indole With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.5h; Inert atmosphere; Sealed tube; Stage #2: methyl halide In tetrahydrofuran; mineral oil at 0 - 20℃; Inert atmosphere; Sealed tube; | 99% |
| Stage #1: indole With sodium hydroxide In dimethyl sulfoxide Stage #2: methyl halide In dimethyl sulfoxide at 10 - 15℃; for 3h; | |
| Stage #1: indole With sodium hydride In N,N-dimethyl-formamide at 0 - 23℃; for 0.5h; Inert atmosphere; Stage #2: methyl halide In N,N-dimethyl-formamide at 0 - 23℃; Inert atmosphere; |

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate; water In chloroform at 80℃; for 24h; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonium chloride at 150 - 170℃; under 12001.2 - 24002.4 Torr; Inert atmosphere; Sealed tube; Autoclave; | 98.8% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50 - 130℃; for 9h; | 98% |
| With tributyl-amine In N,N-dimethyl-formamide at 285℃; under 112511 Torr; for 0.05h; | 98% |
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 94 - 95℃; for 8h; | 97% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; | 97% |
| Stage #1: indole With potassium hydroxide In N,N-dimethyl-formamide at 0℃; for 0.333333h; Stage #2: methyl bromide In N,N-dimethyl-formamide at 0 - 20℃; for 1h; | |
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 24h; | |
| Stage #1: indole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: methyl bromide In N,N-dimethyl-formamide at 0℃; | |
| With potassium hydroxide In N,N-dimethyl-formamide at 20℃; for 24h; Inert atmosphere; |
-
-
590417-55-3
4-bromo-1-methyl-1H-indole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; benzyl alcohol In N,N-dimethyl-formamide at 130℃; for 2.5h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 4h; Sealed tube; Glovebox; | 96% |
| In acetonitrile at 20℃; | 96% |
-
-
112398-75-1
5-chloro-1-methyl-1H-indole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; N,N-dimethyl-formamide In dimethyl sulfoxide at 35℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 96% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In acetone at 20℃; for 1h; | 94% |
| With potassium hydroxide In acetone Methylation; | 93% |
| With sodium amide; toluene | |
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| With p-benzoquinone; lithium chloride; dichloro bis(acetonitrile) palladium(II) In tetrahydrofuran for 18h; Heating; | 94% |
-
-
19012-03-4
N-methyl-3-formylindole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With palladium nanoparticles supported on fibrous silica In cyclohexane at 130℃; for 22h; Molecular sieve; | 94% |

| Conditions | Yield |
|---|---|
| With dimethylethylsilane; 2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; bis(pentafluorophenyl)borohydride In dichloromethane; toluene at 20℃; for 24h; Inert atmosphere; Sealed tube; chemoselective reaction; | 94% |
-
-
81471-20-7
3-bromo-1-methyl-1H-indole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran at 20℃; for 19h; Reagent/catalyst; Time; Inert atmosphere; | 93% |
| With di-tert-butyl peroxide; caesium carbonate; isopropyl alcohol at 120℃; for 24h; Inert atmosphere; | 90 %Chromat. |
-
-
81471-20-7
3-bromo-1-methyl-1H-indole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; sodium tetrahydroborate; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran at 25℃; for 19h; Inert atmosphere; | 93% |
-
-
280563-07-7
1-methyl-5-iodoindole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; benzyl alcohol In N,N-dimethyl-formamide at 90℃; for 2h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 92% |
| With potassium tert-butylate; benzaldehyde In N,N-dimethyl-formamide at 90℃; for 2h; Schlenk technique; Inert atmosphere; | 86% |
| With 1,3-bis(2,6-diisopropylphenyl)-2,4-diphenylimidazole In N,N-dimethyl-formamide at 20℃; for 36h; Glovebox; | 85% |
| With 2-(2-methoxyethoxy)ethyl alcohol; oxygen; sodium hydride In 1,4-dioxane at 20℃; for 24h; Schlenk technique; chemoselective reaction; | 76% |
-
-
10075-52-2
5-bromo-1-methyl-H-indole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With 1,1,1,2,2,2-hexamethyldisilane; potassium methanolate; acetonitrile at 25℃; for 5h; Reagent/catalyst; Inert atmosphere; Glovebox; Sealed tube; | 92% |
| With 2-H-1,3-di-tert-butyl-1,3,2-diazaphosphorinane; 2,2'-azobis(isobutyronitrile) In toluene at 90℃; for 12h; | 70% |
| With tributyl-amine; tetrabutylammonium tetrafluoroborate In acetonitrile at 20℃; for 1.8h; Inert atmosphere; Electrolysis; | 64% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 4h; Solvent; Sealed tube; Glovebox; | 92% |
| In acetonitrile at 20℃; Solvent; | 95 %Chromat. |
-
-
32387-21-6
1-methyl-3-indolecarboxylic acid

-
-
91-01-0
1,1-Diphenylmethanol

-
A
-
53924-28-0
3-(1,1-diphenylmethyl)-1-methyl-1H-indole

-
B
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With sodium tetrachloroaurate(III) dihyrate; sodium 3-(diphenylphosphanyl)benzenesulfonate In water at 120℃; for 16h; Reagent/catalyst; Solvent; Sealed tube; | A 91% B 9% |
| With sodium tetrachloroaurate(III) dihyrate; sodium 3-(diphenylphosphanyl)benzenesulfonate In water at 120℃; for 16h; Reagent/catalyst; Solvent; Sealed tube; | A 5% B 47% |
| With sodium tetrachloroaurate(III) dihyrate; sodium 3-(diphenylphosphanyl)benzenesulfonate In water at 120℃; for 16h; Reagent/catalyst; Solvent; Sealed tube; | A 47% B 5% |


| Conditions | Yield |
|---|---|
| Stage #1: indole With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: methy halide at 0 - 20℃; for 24h; | 90% |

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 3h; | 90% |
-
-
219605-52-4
2′-N,N-dimethylaminophenylacetylene

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With ethanol at 120℃; for 4h; Microwave irradiation; Inert atmosphere; Green chemistry; | 89% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide for 0.166667h; Heating; | 88% |
| With potassium tert-butylate In N,N-dimethyl-formamide for 0.25h; Heating; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: indole With potassium tert-butylate In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Stage #2: trifluoroacetic acid-methyl ester In N,N-dimethyl-formamide for 10h; | 88% |
-
-
89246-30-0
2-bromo-1-methyl-1H-indole

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; sodium tetrahydroborate; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran at 25℃; for 0.8h; Reagent/catalyst; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With copper(II) choride dihydrate; oxygen In dimethyl sulfoxide at 20 - 120℃; for 0.166667h; | 87% |
| Multi-step reaction with 2 steps 1: sodium hydride / tetrahydrofuran; mineral oil / 24 h / 20 °C 2: tris(pentafluorophenyl)borate / para-xylene / 22 h / 150 °C / Inert atmosphere; Sealed tube View Scheme | |
| Multi-step reaction with 2 steps 1: triethylamine / dimethyl sulfoxide / 20 h / 150 °C / Sealed tube; Schlenk technique; Inert atmosphere 2: sodium carbonate / ethyl acetate / 24 h / 120 °C / Sealed tube; Green chemistry View Scheme | |
| Multi-step reaction with 2 steps 1: acetic acid; sodium cyanoborohydride / water; methanol / 1 h / 20 °C 2: dichloro[1,3-bis(2-methylphenyl)-2-imidazolidinylidene](benzylidene) (tricyclohexylphosphine) ruthenium(II); oxygen / ethyl acetate / 46 h / 70 °C / 760.05 Torr / Sealed tube View Scheme |

| Conditions | Yield |
|---|---|
| 100% |
-
-
603-76-9
1-methylindole

-
-
122-51-0
orthoformic acid triethyl ester

-
-
27065-95-8
tri(1-methyl-1H-indole-3-yl)methane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In methanol at 70℃; for 0.166667h; | 100% |
| With iodine In acetonitrile at 20℃; for 0.133333h; | 90% |
| With acid-washed montmorillonite K10 clay at 20℃; | 87% |
| With acid-washed montmorillonite K10 clay (acid-clay) at 20℃; |
-
-
603-76-9
1-methylindole

-
-
84-58-2
2,3-dicyano-5,6-dichloro-p-benzoquinone

-
-
61995-39-9
4,5-Dichloro-3-hydroxy-1-(1-methyl-1H-indol-3-yl)-6-oxo-cyclohexa-2,4-diene-1,2-dicarbonitrile

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 24h; Ambient temperature; | 100% |
-
-
603-76-9
1-methylindole

-
-
85092-84-8
3-iodo-1-methyl-1H-indole

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In N,N-dimethyl-formamide at 0℃; for 1.5h; Inert atmosphere; | 100% |
| With potassium hydroxide; iodine In N,N-dimethyl-formamide Ambient temperature; | 98% |
| With iodine; potassium hydroxide In N,N-dimethyl-formamide at 20℃; for 0.166667h; Inert atmosphere; Schlenk technique; Glovebox; | 97% |
-
-
603-76-9
1-methylindole

-
-
5153-67-3
nitrostyrene

-
-
109811-96-3
1-methyl-3-(2-nitro-1-phenylethyl)-1H-indole

| Conditions | Yield |
|---|---|
| With Zn(2+)*2C6H6NO2S2(1-) In ethanol at 20℃; for 24h; | 100% |
| With 1H-pyrrolo[2,3-b]pyridinium substituted-borate salt In toluene at 0 - 20℃; for 24h; | 99% |
| Stage #1: nitrostyrene With (S)-10,10'-bis[(S)-4-isopropyl-4,5-dihydrooxazol-2-yl]-9,9'-biphenanthrene; zinc(II) trifluoroacetate In diethyl ether at 20℃; for 0.25h; Inert atmosphere; Stage #2: 1-methylindole In diethyl ether at 20℃; Friedel-Crafts alkylation; Inert atmosphere; | 99% |
-
-
603-76-9
1-methylindole

-
-
78-94-4
methyl vinyl ketone

-
-
91956-43-3
4-(1-methyl-1H-indol-3-yl)-butan-2-one

| Conditions | Yield |
|---|---|
| With trityl tetrafluoroborate In dichloromethane Inert atmosphere; | 100% |
| With rac-9-phenyl-9H-cyclopenta[1,2-c:4,3-c']diphenanthren-9-ylium tetrakis(3,5-bis(tri-fluoromethyl)phenyl)borate In dichloromethane at 20℃; for 24h; Friedel-Crafts Alkylation; Glovebox; Inert atmosphere; | 100% |
| hafnium tetrachloride In acetonitrile at 20℃; for 0.333333h; | 99% |

| Conditions | Yield |
|---|---|
| With o-benzenedisulfonimide at 20℃; for 0.0833333h; Friedel Crafts alkylation; neat (no solvent); | 100% |
| With cellulose sulfuric acid at 50℃; for 0.333333h; Neat (no solvent); Grinding; Combinatorial reaction / High throughput screening (HTS); chemoselective reaction; | 98% |
| With N-Bromosuccinimide at 20℃; for 6h; | 96% |
-
-
603-76-9
1-methylindole

-
-
62668-02-4
(E)-1,3-diphenyl-2-propen-1-ol

| Conditions | Yield |
|---|---|
| With 5,11,17,23-tetrasulfonate-25,26,27,28-tetrakis(propoxy)calix[4]arene tetrasodium salt In water at 50℃; for 61h; Reagent/catalyst; Concentration; Time; Green chemistry; | 100% |
| With magnetic Fe3O4-grafted calix[6]arene sulfonic acid In water at 50℃; for 24h; Catalytic behavior; Reagent/catalyst; | 99.9% |
| With zinc dibromide In neat (no solvent) for 1.5h; Milling; Green chemistry; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With 5,11,17,23-tetrasulfonate-25,26,27,28-tetrakis(propoxy)calix[4]arene tetrasodium salt In water at 50℃; for 24h; Reagent/catalyst; Concentration; Green chemistry; | 100% |
| With magnetic Fe3O4-grafted calix[4]arene sulfonic acid In water at 50℃; for 25h; Catalytic behavior; Reagent/catalyst; | 99.9% |
| With sodium tetrachloroaurate(III) dihydrate; sodium 3-(diphenylphosphanyl)benzenesulfonate In water at 80℃; for 16h; Sealed tube; | 95% |
| With o-benzenedisulfonimide In neat (no solvent) at 20℃; for 0.25h; Friedel-Crafts Alkylation; | 94% |
| With dodecylbenzene-sulphonic acid In water at 80℃; for 24h; Friedel-Crafts reaction; | 92% |

| Conditions | Yield |
|---|---|
| With indium(III) bromide In dichloromethane at 20℃; for 2h; Michael addition; diastereoselective reaction; | 100% |
-
-
603-76-9
1-methylindole

-
A
-
1283232-87-0
3-(9-borabicyclo[3.3.1]nonan-9-yl)-1-methyl-1H-indole

| Conditions | Yield |
|---|---|
| In dichloromethane at 50℃; for 2h; Sealed vessel; Inert atmosphere; | A 100% B n/a |

-
-
603-76-9
1-methylindole

-
-
3140-75-8
4,4′,4″‑[(1,3,5‑triazine‑2,4,6‑triyl)tris(oxy)]tribenzaldehyde

-
-
1431159-05-5
C78H63N9O3

| Conditions | Yield |
|---|---|
| With silica sulfuric acid In acetonitrile at 20℃; for 0.5h; Friedel-Crafts Alkylation; | 100% |
-
-
860772-38-9
(E)‐1‐(1‐methyl‐1H‐imidazole‐2‐yl)‐but‐2‐en‐1‐one

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With [ZnIIYIII(N,N'-bis(3-methoxysalicylidene)cyclohexane-1,2-diamine)(NO3)2(o-vanillin)2(MeOH)](MeOH) In ethyl acetate at 20℃; for 72h; Friedel-Crafts Alkylation; Inert atmosphere; | 100% |
| With copper(II) nitrate trihydrate In aq. buffer at 20℃; for 72h; pH=6.5; Friedel-Crafts Alkylation; | 88% |
| With copper(II) nitrate trihydrate In aq. buffer at 20℃; for 72h; pH=6.5; Reagent/catalyst; Friedel-Crafts Alkylation; enantioselective reaction; | 88% |
| With 4,4'-dimethyl-2,2'-bipyridines; copper(II) nitrate trihydrate In acetonitrile at 20℃; for 72h; Catalytic behavior; Reagent/catalyst; Friedel-Crafts Alkylation; Inert atmosphere; | 80% |
| With copper(II) nitrate trihydrate In acetonitrile at 20℃; for 16h; pH=7; Friedel-Crafts Alkylation; |

| Conditions | Yield |
|---|---|
| With iron(III) chloride In 1,4-dioxane at 20℃; for 6h; | 100% |
-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With dimethylaluminum chloride In hexane; toluene at -30℃; for 18h; Temperature; | 100% |

| Conditions | Yield |
|---|---|
| With norborn-2-ene; carbonyl bis(hydrido)tris(triphenylphosphine)ruthenium(II) In toluene at 150℃; for 16h; Inert atmosphere; | 100% |
-
-
868765-12-2
2-phenylethynyl-quinoline-3-carbaldehyde

-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With silver trifluoromethanesulfonate In 1,2-dichloro-ethane for 1 - 24h; Inert atmosphere; Reflux; | 100% |
-
-
603-76-9
1-methylindole

-
-
1421925-29-2
7-chloro-2-(phenylethynyl)quinoline-3-carbaldehyde

| Conditions | Yield |
|---|---|
| With silver trifluoromethanesulfonate In 1,2-dichloro-ethane for 1 - 24h; Inert atmosphere; Reflux; | 100% |
-
-
603-76-9
1-methylindole

-
-
1170697-31-0
1-methyl-3-propyl-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-3-propyl-1H-indole With 2,6-di-tert-butyl-pyridine; trifluoromethylsulfonic anhydride; dimethyl sulfoxide In dichloromethane; dimethyl sulfoxide at -78℃; for 0.25h; Inert atmosphere; Stage #2: 1-methylindole In dichloromethane; dimethyl sulfoxide for 0.25h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With C24H30N8; scandium tris(trifluoromethanesulfonate) In acetonitrile at 20℃; for 18h; Friedel-Crafts Alkylation; Inert atmosphere; Molecular sieve; chemoselective reaction; | 100% |
-
-
603-76-9
1-methylindole

-
-
706-08-1, 5153-69-5
trans-1-(4-fluorophenyl)-2-nitro-ethene

-
-
1430076-20-2
3-(1-(4-fluorophenyl)-2-nitroethyl)-1-methyl-1H-indole

| Conditions | Yield |
|---|---|
| With Zn(2+)*2C6H6NO2S2(1-) In ethanol at 20℃; for 24h; | 100% |
| With C22H35N4S(1+)*BF4(1-) In dichloromethane at 20℃; for 44h; Friedel-Crafts Alkylation; Inert atmosphere; | 92% |
| With rose bengal In water at 60℃; Friedel-Crafts Alkylation; Irradiation; | 81% |
| With 2C32H12BF24(1-)*C13H16N4S(2+) In chloroform-d1 at 20℃; Inert atmosphere; |
-
-
603-76-9
1-methylindole

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In tetrahydrofuran; methanol at 20℃; diastereoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; 1,1,1,3',3',3'-hexafluoro-propanol at 60℃; for 24h; Reagent/catalyst; | 100% |
-
-
603-76-9
1-methylindole

-
-
50-00-0
formaldehyd

-
-
124-40-3
dimethyl amine

-
-
52972-61-9
3-(N,N-dimethylaminomethyl)-1-methylindole

| Conditions | Yield |
|---|---|
| Stage #1: 1-methylindole; formaldehyd; dimethyl amine With acetic acid In water at 0 - 50℃; Mannich reaction; Stage #2: With sodium hydroxide In water | 99% |
| With acetic acid at 0 - 50℃; for 14h; | 91% |
| With zinc(II) chloride In ethanol at 20℃; for 2.5h; Mannich reaction; | 82% |

-
-
603-76-9
1-methylindole

-
-
2058-74-4
1-methyl-1H-indole-2,3-dione

-
-
75833-69-1
1-methyl-3,3-bis(1-methyl-1H-indol-3-yl)-2,3-dihydro-1H-indol-2-one

| Conditions | Yield |
|---|---|
| With C19H22N4; scandium tris(trifluoromethanesulfonate) In acetonitrile at 20℃; for 18h; Friedel-Crafts Alkylation; Inert atmosphere; Molecular sieve; chemoselective reaction; | 99% |
| In acetic acid at 35℃; for 24h; | 92% |
| With H6P2W18O62 In water at 60℃; for 0.5h; | 90% |

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid In diethyl ether; dichloromethane for 0.5h; | 99% |
-
-
603-76-9
1-methylindole

-
-
13887-54-2
methyl 2-methoxy-2-(piperidin-1-yl)acetate

| Conditions | Yield |
|---|---|
| With hafnium tetrakis(trifluoromethanesulfonate); chloro-trimethyl-silane In dichloromethane at 20℃; for 1h; | 99% |
| With Methyltrichlorosilane In acetonitrile for 39h; Ambient temperature; | 88% |
-
-
603-76-9
1-methylindole

-
-
1147550-11-5
ammonium thiocyanate

-
-
23518-17-4
N-methyl-3-thiocyanato-1H-indole

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In methanol Ambient temperature; | 99% |
| In acetonitrile at 20℃; for 3h; Electrochemical reaction; Inert atmosphere; | 99% |
| With oxygen In tetrahydrofuran at 25℃; under 750.075 Torr; for 10h; Irradiation; | 98% |
1-Methylindole Chemical Properties
MF: C9H9N
MW: 131.17
EINECS: 210-057-5
BP: 133 °C26 mm Hg
Density : 1.051 g/mL at 20 °C
Refractive Index : n20/D 1.606
FP >230 °C
Storage temp : Refrigerator (+4°C)
Water Solubility : insoluble
Sensitive : Light Sensitive
BRN : 111026
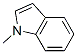
Structural Formula of 1-Methylindole(603-76-9):
1-Methylindole Toxicity Data With Reference
 Xi
Xi Risk Statements :
R36/37/38: Irritating to eyes, respiratory system and skin
1-Methylindole Safety Profile
S22: Do not breathe dust
S24/25: Avoid contact with skin and eyes
S37/39: Wear suitable gloves and eye/face protection
S26: In case of contact with eyes, rinse immediately with plenty of WATER and seek medical advice
1-Methylindole Specification
Conditions to Avoid: Incompatible materials, light.
Incompatibilities with Other Materials Strong oxidizing agents.
Hazardous Decomposition Products Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization Has not been reported.
Related Products
- 1-Methylindole
- 1-Methylindole-2-boronic acid
- 1-Methylindole-2-carboxylic acid
- 1-Methylindole-3-acetic acid
- 1-Methylindole-3-acetonitrile
- 1-Methylindole-3-carboxaldehyde
- 1-Methylindole-3-carboxylic acid
- 1-Methylindole-5-boronic acid
- 1-Methylindole-5-boronic acid pinacol ester
- 60376-97-8
- 60379-01-3
- 603-79-2
- 603-80-5
- 603-81-6
- 6038-19-3
- 603-83-8
- 60384-53-4
- 603-84-9
- 603-85-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View