-
Name
1-Nonanal
- EINECS 204-688-5
- CAS No. 124-19-6
- Article Data548
- CAS DataBase
- Density 0.816 g/cm3
- Solubility practically insoluble in water
- Melting Point -18oC
- Formula C9H18O
- Boiling Point 190.778 °C at 760 mmHg
- Molecular Weight 142.241
- Flash Point 63.889 °C
- Transport Information UN 3082
- Appearance brown liquid
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Nonanaldehyde(7CI);Aldehyde C 09;Aldehyde C 9;NSC 5518;Nonaldehyde;Nonanoic aldehyde;Nonylaldehyde;Nonylic aldehyde;Pelargonaldehyde;Pelargonic aldehyde;n-Nonanal;n-Nonylaldehyde;Nonanal;
- PSA 17.07000
- LogP 2.93590
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: Methyl oleate With ozone In dichloromethane at -78℃; Stage #2: With triphenylphosphine In dichloromethane at -78 - 23℃; for 18h; | A 100% B 100% |
| With N-methyl-2-indolinone; ozone at 0℃; | A 74% B 96% |
| With ozone; acetic acid; zinc 1.) MeOH, CH2Cl2, -78 deg C, 2.) MeOH, CH2Cl2, 30 min; Multistep reaction. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With 2-pyridylpropylimine rhodium(I)chlorocarbonyl; hydrogen In toluene at 95℃; Catalytic behavior; Reagent/catalyst; Pressure; Temperature; Autoclave; chemoselective reaction; | 100% |
| With hydrogen In dichloromethane at 45℃; under 51716.2 Torr; for 16h; Autoclave; Inert atmosphere; Green chemistry; regioselective reaction; | 100% |
| With hydrogen In cyclohexane at 120℃; under 37503.8 Torr; for 4h; Autoclave; | 99% |

| Conditions | Yield |
|---|---|
| With sodium periodate In 1,4-dioxane; water at 20℃; for 1h; | A n/a B 100% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 100℃; under 15001.5 Torr; for 12h; Autoclave; Green chemistry; regioselective reaction; | 99.5% |
| With hydrogen; 4,5-bis(9-dibenzo[b,d]phospholyl)-2,7-di-tert-butyl-9,9-dimethylxanthene; (2,2,6,6-tetramethyl-3,5-heptanedionate)Rh(CO)2 In toluene at 80℃; under 15001.2 Torr; for 1h; Yield given; | |
| With hydrogen; 2,2'-bis(di(3,4,5-F-phenyl)phosphanylmethyl)-1,1'-binaphthyl; acetylacetonatodicarbonylrhodium(l) In methoxybenzene; toluene at 120℃; under 7500.6 Torr; for 16h; |

| Conditions | Yield |
|---|---|
| With 4-methoxy-TEMPO; sodium hypochlorite; potassium bromide In dichloromethane; water at 0℃; for 0.05h; Mechanism; Product distribution; pH = 8.6; other primary and secondary alcohols, var. temp., time, pH, and catalytic species; | 98% |
| With tert.-butylhydroperoxide In water at 60℃; for 3h; | 98% |
| With 4-methoxy-TEMPO; sodium hypochlorite; potassium bromide In dichloromethane; water at 0℃; for 0.05h; pH = 8.6; | 92% |
-
-
39854-46-1
2-(n-Octyl)-1,3-dithiane

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With tri(p-tolyl)amine; lithium perchlorate; sodium hydrogencarbonate In water; acetonitrile at 20℃; electrolysis; | 97% |
| With periodic acid In tetrahydrofuran; diethyl ether for 0.0833333h; Ambient temperature; | 91% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In dichloromethane at 25℃; for 2.5h; Inert atmosphere; | 97% |
| With sodium periodate; sodium hydrogencarbonate In water; acetonitrile at 20℃; for 1.5h; | |
| With N-Bromosuccinimide; (oxybis(2,1-phenylene))bis(diphenylbismuthane); potassium carbonate In [D3]acetonitrile at 23℃; for 1h; Schlenk technique; | 70 %Spectr. |

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With hydrogen fluoride In acetonitrile at 20℃; | 96% |
-
-
2566-91-8
epoxidized methyl oleate

-
A
-
124-19-6
nonan-1-al

-
B
-
1931-63-1
methyl ester of azelaic acid aldehyde

| Conditions | Yield |
|---|---|
| With periodic acid In diethyl ether | A n/a B 95% |
| With periodic acid for 0.05h; Product distribution; Ambient temperature; | |
| Multi-step reaction with 2 steps 1: sulfuric acid / acetic acid; acetonitrile; water / 16 h / 20 °C 2: sodium periodate; sodium hydrogencarbonate / acetic acid; acetonitrile; water / 1.5 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With oxygen; ozone; 4-methylmorpholine N-oxide In dichloromethane at 0℃; | 94% |
| With ozone; 4-methylmorpholine N-oxide In dichloromethane at 0 - 20℃; | 94% |
| With sodium periodate; 1,4-diazobicyclo<2.2.2>octane quaternized with chloromethylated styrene-divinylbenzene copolymer*OsO4 In 1,4-dioxane; water for 0.5h; Ambient temperature; | 100 % Chromat. |
-
-
113388-45-7
Formic acid (E)-non-1-enyl ester

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water for 23h; Ambient temperature; | 94% |
-
-
111-66-0
oct-1-ene

-
-
201230-82-2
carbon monoxide

-
A
-
124-19-6
nonan-1-al

-
B
-
49642-49-1, 55352-42-6, 72598-55-1, 7786-29-0
2-methyl-1-octanal

| Conditions | Yield |
|---|---|
| With hydrogen In cyclohexane at 120℃; under 37503.8 Torr; for 3h; Autoclave; | A 93% B 7% |
| With hydrogen; 1-octyl-3-methyl-imidazolium bromide In water at 100℃; under 15001.5 Torr; for 3h; Product distribution; Further Variations:; Reagents; reaction time; | A 91.5% B 5.7% |
| With hydrogen In n-heptane; toluene at 90℃; under 37503.8 Torr; for 20h; | A n/a B 91% |

-
-
56888-88-1
2-methyl-undec-2-ene

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With ozone In dichloromethane at -78℃; | 93% |
| With ozone In dichloromethane at -78℃; | 93% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 10h; Ambient temperature; | 92% |
| With ammonium cerium(IV) nitrate In methanol; water at 20℃; for 0.166667h; Ring cleavage; | 91% |
| With dimethylboron bromide; sodium hydrogencarbonate 1) CH2Cl2, -78 deg C, 1h, 2) THF, 5 min; Yield given. Multistep reaction; |
-
-
111-66-0
oct-1-ene

-
-
201230-82-2
carbon monoxide

-
A
-
124-19-6
nonan-1-al

-
B
-
111-65-9
octane

-
C
-
111-67-1
2-octene

| Conditions | Yield |
|---|---|
| With (acetylacetonato)dicarbonylrhodium (l); 2,2'-bis((dipyrrolylphosphinooxy)methyl)-1,1'-(±)-biphenyl; hydrogen In toluene at 80℃; under 11251.1 Torr; for 1h; Pressure; Reagent/catalyst; Temperature; regioselective reaction; | A 91.9% B n/a C n/a |

-
-
18824-63-0
1,1-dimethoxynonane

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With dimethylboron bromide In dichloromethane; 1,2-dichloro-ethane at -78℃; for 1h; | 91% |
| With guanidine hydrochloride; acetyl chloride for 3h; Ambient temperature; | 85% |
| With diphosphorus tetraiodide; propene In dichloromethane at 20℃; for 0.2h; | 64% |
-
-
18388-84-6
trimethylsilyloxy-nonane

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate; HZSM-5 zeolite In water for 0.133333h; microwave irradiation; | 91% |
| With chromium(VI) oxide In pyridine; dichloromethane at 0℃; for 1h; |

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With periodic acid In tetrahydrofuran; diethyl ether for 0.166667h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: cis-Octadecenoic acid With ozone In dichloromethane at -78℃; Stage #2: With dimethylsulfide In dichloromethane at 20℃; for 3h; | A n/a B 90% |
| Stage #1: cis-Octadecenoic acid With ozone at 95℃; Stage #2: With hydrogen at 30℃; under 3750.38 Torr; palladium-coated film reactor; | A n/a B 48.7% |
| With 1-carboxymethyl-3-methylimidazol-3-ium hydrogen sulfate; dihydrogen peroxide at 5℃; for 5h; | |
| Multi-step reaction with 3 steps 1: [((S,S)-N,N′-bis(2-pyridylmethyl)-(S,S)-2,2′-bipyrrolidine)FeII(OTf)2]; dihydrogen peroxide / acetonitrile / 2.5 h / 0 °C 2: sulfuric acid / acetonitrile; water / 16 h / 20 °C 3: sodium periodate; sodium hydrogencarbonate / acetonitrile; water / 1.5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium hydroxide; potassium permanganate / water / 0.25 h / 0 - 50 °C 2: sodium periodate; tetra(n-butyl)ammonium hydrogen sulfate / water; dichloromethane / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In water; toluene at 90℃; for 0.5h; Microwave irradiation; Sealed vial; | 90% |
-
-
112-62-9
octadec-9-enoic acid methyl ester

-
A
-
124-19-6
nonan-1-al

-
B
-
1931-63-1
methyl ester of azelaic acid aldehyde

| Conditions | Yield |
|---|---|
| With ozone; triphenylphosphine In dichloromethane at -78℃; for 24h; Inert atmosphere; | A 90% B 75% |
| With ozone; potassium iodide In water; tert-butyl alcohol for 1h; | |
| Stage #1: octadec-9-enoic acid methyl ester With oxygen; ozone In neat (no solvent) at 25℃; Stage #2: With 5%-palladium/activated carbon; hydrogen at 20℃; for 1h; Solvent; Temperature; |

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide at 20℃; for 17h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium periodate; phosphoric acid; sodium carbonate | A 61.5% B 89% |
| With sodium periodate; sodium hydrogencarbonate In water; acetic acid; acetonitrile at 20℃; for 24h; |
-
-
16742-11-3
nonanal semicarbazone

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| With K-10 clay-supported Fe(NO3)3 ("clayfen" reagent) In dichloromethane for 1.16667h; Ambient temperature; | 89% |

| Conditions | Yield |
|---|---|
| With water; ruthenium-based phosphine complex In acetone at 120℃; for 26h; | 89% |
| With [CpRu(6-Ph2P-Py-2-yl)(MeCN)][PF6(1-)]; water In acetone at 70℃; for 3h; | 99.9 % Spectr. |
| With water; [cyclopentadienylruthenium(II) bis(2-diphenylphosphino-6-t-butylpyridine)(acetonitrile)]CF3SO3 In acetone at 70℃; Kinetics; |

| Conditions | Yield |
|---|---|
| With 3-butyl-4,5-dimethylthiazol-3-ium trifluoromethanesulfonate; potassium carbonate at 180℃; under 7.50075 Torr; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In dichloromethane at -78℃; for 0.5h; Inert atmosphere; | 88% |
-
-
13389-42-9
trans-2-Octene

-
-
201230-82-2
carbon monoxide

-
A
-
124-19-6
nonan-1-al

-
B
-
27649-40-7
2-ethylheptanal

-
C
-
49642-49-1, 55352-42-6, 72598-55-1, 7786-29-0
2-methyl-1-octanal

-
D
-
140238-46-6, 140238-47-7, 27644-47-9
2-n-propylhexanal

| Conditions | Yield |
|---|---|
| With zinc 5,10,15,20-tetraphenylporphyrin; N-ethyl-N,N-diisopropylamine; tri(pyridin-3-yl)phosphine; acetylacetonatodicarbonylrhodium(l) In toluene at 25℃; for 73h; Product distribution; Further Variations:; Catalysts; Temperatures; | A 0.7% B 87.8% C 9.4% D 0.5% |
| With 25,26,27,28-tetrakis(hydroxy)calix[4]arene; hydrogen; phosphan; acetylacetonatodicarbonylrhodium(l) In water at 140℃; under 30003 Torr; for 12h; Product distribution; | |
| With (acetylacetonato)dicarbonylrhodium (l); rac-3-methyl-2-(2-methylnaphthalen-1-yl)-4-phenyl-5,6-dihydrobenzo[1,2-h]phosphinoline; hydrogen In toluene at 80℃; under 15001.5 Torr; for 90h; Reagent/catalyst; Autoclave; |

-
-
14850-23-8
trans-4-Octene

-
-
75-09-2
dichloromethane

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| Stage #1: trans-4-Octene; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane With triphenylphosphine; chlorobis(ethylene)rhodium(I) dimer In dichloromethane for 0.5h; Stage #2: dichloromethane With n-butyllithium In tetrahydrofuran at -78 - 20℃; Stage #3: With dihydrogen peroxide; sodium carbonate In tetrahydrofuran; water at 0 - 20℃; | 86% |

-
-
7642-04-8
cis-2-octene

-
-
75-09-2
dichloromethane

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| Stage #1: cis-2-octene; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane With triphenylphosphine; chlorobis(ethylene)rhodium(I) dimer In dichloromethane for 0.5h; Stage #2: dichloromethane With n-butyllithium In tetrahydrofuran at -78 - 20℃; Stage #3: With dihydrogen peroxide; sodium carbonate In tetrahydrofuran; water at 0 - 20℃; | 86% |


| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride In methanol; diethyl ether for 4h; Reduction; Heating; | 100% |
| With hydrogenchloride; tetrahydrogenoboratebis(triphenylphosphine)copper(I) In dichloromethane | 94% |
| With hydrogenchloride; samarium In tetrahydrofuran at 20℃; | 94% |

| Conditions | Yield |
|---|---|
| at 25℃; | 100% |

| Conditions | Yield |
|---|---|
| With indium In water at 20℃; for 3h; Addition; | 100% |
| With indium In N,N-dimethyl-formamide for 3h; Ambient temperature; | 93% |
-
-
124-19-6
nonan-1-al

-
-
496922-59-9
N-benzyl-N-(1-octynyl)-p-toluenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: N-benzyl-N-(1-octynyl)-p-toluenesulfonamide With titanium(IV) isopropylate; isopropylmagnesium chloride Stage #2: nonan-1-al Stage #3: With hydrogen cation Acid hydrolysis; Further stages.; | 100% |
-
-
124-19-6
nonan-1-al

-
-
501-65-5
diphenyl acetylene

-
-
205885-39-8
N-benzyl-N-ethynyl-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: diphenyl acetylene With titanium(IV) isopropylate; isopropylmagnesium bromide In diethyl ether at -78 - -50℃; for 2.5h; Stage #2: N-benzyl-N-ethynyl-4-methyl-benzenesulfonamide In diethyl ether at -50℃; for 4h; Stage #3: nonan-1-al In diethyl ether at -50 - 20℃; | 100% |
| Multistep reaction.; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: acetoacetic acid methyl ester With sodium hydride In tetrahydrofuran at 0℃; for 0.166667h; Stage #2: With n-butyllithium In tetrahydrofuran; hexane at 20℃; for 0.333333h; Stage #3: nonan-1-al In tetrahydrofuran; hexane at -78 - 20℃; for 1.08333h; | 100% |
| Stage #1: acetoacetic acid methyl ester With sodium hydride In tetrahydrofuran; mineral oil at 0 - 5℃; for 0.166667h; Stage #2: With n-butyllithium In tetrahydrofuran; mineral oil at 5℃; for 0.166667h; Stage #3: nonan-1-al In tetrahydrofuran; mineral oil at 5 - 10℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With anthranilic acid In dichloromethane at 60℃; for 24h; Molecular sieve; | 100% |
-
-
124-19-6
nonan-1-al

| Conditions | Yield |
|---|---|
| Stage #1: (3S)-5,7-dimethoxy-4-methyl-3-(1-oxopropyl)phthalide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: nonan-1-al In tetrahydrofuran at -78℃; for 2h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With Oxone In N,N-dimethyl-formamide at 20℃; for 3h; | 99% |
| With Oxone; ethylenediaminetetraacetic acid; sodium hydrogencarbonate In water; acetone at 22℃; for 1.5h; Oxidation; | 86% |
| With NAD; Geotrichum candidum aldehyde dehydrogenase In aq. buffer at 40℃; for 3h; pH=7.2; Green chemistry; Enzymatic reaction; | 43% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran | 99% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at -78 - 20℃; Inert atmosphere; | 99% |
| With magnesium bromide diethyl etherate In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; | 90% |
| In tetrahydrofuran 1.) RT, overnight, 2.) reflux, 6 h; | 84% |
-
-
124-19-6
nonan-1-al

-
-
50603-71-9
3-methoxycarbonyl-2H-cyclohepta[b]furan-2-one

| Conditions | Yield |
|---|---|
| With morpholine In ethanol for 4h; Heating; | 99% |
-
-
124-19-6
nonan-1-al

-
-
1066-54-2
trimethylsilylacetylene

-
-
74794-25-5, 132958-42-0, 61077-68-7
(+/-)-1-trimethylsilyl-1-undecyn-3-ol

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsilylacetylene With n-butyllithium In tetrahydrofuran; hexane at -75 - -10℃; for 0.5h; Inert atmosphere; Stage #2: nonan-1-al In tetrahydrofuran; hexane at -78 - 20℃; for 1h; | 99% |
| With ethylmagnesium bromide In tetrahydrofuran for 2h; Heating; | 74% |
| With n-butyllithium In tetrahydrofuran; hexane 1.) -85 deg C, 15 min, 2.) -85 deg C to 0 deg C, 2 h; | |
| Stage #1: trimethylsilylacetylene With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.5h; Inert atmosphere; Stage #2: nonan-1-al In tetrahydrofuran; hexane at -78℃; for 0.5h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With 1,2-dichloro-benzene at 140 - 170℃; for 0.166667h; | 99% |
-
-
124-19-6
nonan-1-al

-
-
24850-33-7
allyltributylstanane

-
-
85029-09-0, 88691-77-4, 77383-04-1
dodec-1-en-4-ol

| Conditions | Yield |
|---|---|
| With 4-nitro-benzoic acid In acetonitrile at 25℃; for 10h; | 99% |
| With cerium(III) chloride; sodium iodide In acetonitrile at 20℃; for 22h; | 91% |
| With aluminum oxide; cerium(III) chloride; sodium iodide at 50℃; for 18h; | 85% |

| Conditions | Yield |
|---|---|
| With 1,3-dimethylbenzimidazolium Iodide; 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane for 3h; Heating; | 99% |
| With C15H22N3O2(1+)*Cl(1-); 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 0℃; for 5h; Inert atmosphere; | 79% |
| With 3-ethyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide; triethylamine In ethanol at 80℃; for 4h; | 73% |

| Conditions | Yield |
|---|---|
| indium In water Ambient temperature; | 99% |
| With gallium; indium In tetrahydrofuran at 25℃; for 1h; | 75% |

| Conditions | Yield |
|---|---|
| In water Ambient temperature; | 99% |
-
-
124-19-6
nonan-1-al

-
-
74-90-8
hydrogen cyanide

-
-
17672-22-9
2-amino-6-methylphenol

-
-
220184-55-4
1-(2-hydroxy-6-methylphenyl)amino-3-methylbutane-1-carbonitrile

| Conditions | Yield |
|---|---|
| ((R)-3,3'-dibromo-1,1'-bi-2-naphtholato){Zr(CN)(N-methylimidazole)((R)-6,6'-dibromo-1,1'-bi-2-naphtholato)}2 In dichloromethane at -45℃; 5 mol % catalyst; | 99% |
| ((R)-3,3'-dibromo-1,1'-bi-2-naphtholato){Zr(CN)(N-methylimidazole)((R)-6,6'-dibromo-1,1'-bi-2-naphtholato)}2 In dichloromethane at -45℃; 5 mol % catalyst; | 94% |


| Conditions | Yield |
|---|---|
| With N,N-dimethylbenzylamine prolinol trimethylsilyl ether; benzoic acid In water at 20℃; for 22h; Michael addition; optical yield given as %ee; enantioselective reaction; | 99% |
| With N,N-dimethylbenzylamine prolinol trimethylsilyl ether; C15H19N2O2(1+)*F6P(1-) In water at 20℃; for 20h; Michael condensation; optical yield given as %ee; enantioselective reaction; | 98% |
| Stage #1: nonan-1-al With C16H27N5OSi; sodium hydrogencarbonate In sodium chloride at 20℃; for 0.166667h; Michael addition; Stage #2: nitrostyrene In sodium chloride at 20℃; for 48h; Michael addition; optical yield given as %ee; diastereoselective reaction; | 59% |

| Conditions | Yield |
|---|---|
| With C36H44N4O4S2; copper(I) bromide In methanol at 20℃; for 36h; Henry reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
| With C13H24Cl2CuN2; triethylamine In tetrahydrofuran; nitromethane at -20℃; for 49h; Henry Nitro Aldol Condensation; enantioselective reaction; | 98% |
| With (R)-N-methyl-1',2',3',4'-tetrahydro-1,1'-bisisoquinoline; copper(l) chloride In di-isopropyl ether at 0℃; for 20h; Henry reaction; optical yield given as %ee; enantioselective reaction; | 75% |
| With C19H17N; copper(l) chloride In 1,2-dichloro-ethane at 0℃; for 48h; Henry reaction; optical yield given as %ee; enantioselective reaction; | 72% |
-
-
124-19-6
nonan-1-al

-
-
356762-89-5
N-(1,1-diphenyl-3-butenyl)amine

| Conditions | Yield |
|---|---|
| at 20℃; for 6h; Aza-Cope Rearrangement; Molecular sieve; | 99% |
-
-
110-00-9
furan

-
-
124-19-6
nonan-1-al

-
-
92622-43-0
<1(R,S),5(R,S)>-6(R,S)-n-octyl-2,7-dioxabicyclo<3.2.0>hept-3-ene

| Conditions | Yield |
|---|---|
| at -20℃; for 9.5h; Irradiation; | 98.6% |

| Conditions | Yield |
|---|---|
| With thionyl chloride In chloroform for 0.5h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| 98% |
1-Nonanal Chemical Properties
IUPAC Name: Nonanal
CAS: 124-19-6
Formula: C9H18O
Molecular Weight of Nonanal (124-19-6): 142.24
Molecular Structure of Nonanal (124-19-6):
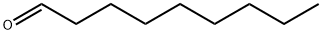
Density: 0.815 g/cm3
Flash Point: 63.9 °C
Boiling Point: 190.8 °C at 760 mmHg
Index of Refraction: 1.417
Molar Refractivity: 43.93 cm3
Molar Volume: 174.4 cm3
Polarizability: 17.41 10-24cm3
Appearance: brown liquid
Surface Tension: 27.4 dyne/cm
Enthalpy of Vaporization: 42.7 kJ/mol
Vapour Pressure: 0.532 mmHg at 25°C
Water Solubility: 131.6(mg/L) at 25°C
1-Nonanal Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H SEV | FCTXAV Food and Cosmetics Toxicology. 11 (1973),1079. | ||
| 2. | sce-rat:lvr 100 nmol/L | MUREAV Mutation Research. 290 (1993),183. | ||
| 3. | msc-ham:lng 100 µmol/L | MUTAEX Mutagenesis. 4 (1989),277. |
1-Nonanal Consensus Reports
Reported in EPA TSCA Inventory.
1-Nonanal Safety Profile
A severe skin irritant. Combustible liquid. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Safety Information about Nonanal (124-19-6):
Hazard Codes:
Xi: 
Risk Statements about Nonanal (124-19-6):
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements about Nonanal (124-19-6):
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39: Wear suitable gloves and eye/face protection.
WGK Germany: 2
HazardClass: 9
PackingGroup: III
1-Nonanal Specification
The chemical synonyms of Nonanal (124-19-6) are Aldehyde c-9 ; 1-Nonanal ; Pelargonic aldehyde ; Pelargonaldehyde ; N-nonylaldehyde ; Nonanal ; Nonanaldehyde . Nonanal (124-19-6) is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Polymerizes readily with sulfuric acid and oxidized to nonanoic acid. This product is sensitive to air,it shoud be stored at 2-8°C.
Related Products
- 1-Nonanal
- 124-20-9
- 124213-39-4
- 124218-96-8
- 124-22-1
- 124221-69-8
- 124222-15-7
- 124222-19-1
- 124222-20-4
- 1242267-80-6
- 1242317-00-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View