-
Name
2-(Trifluoromethoxy)aniline
- EINECS 216-257-9
- CAS No. 1535-75-7
- Article Data5
- CAS DataBase
- Density 1.301 g/cm3
- Solubility
- Melting Point
- Formula C7H6F3NO
- Boiling Point 170.2 °C at 760 mmHg
- Molecular Weight 177.126
- Flash Point 56.7 °C
- Transport Information UN 1993
- Appearance clear, very faintly yellow liquid
- Safety 36/37/39-26-45-16
- Risk Codes 36/37/38-20/21/22-10
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, T,
T, Xi
Xi
- Synonyms o-Anisidine,a,a,a-trifluoro- (6CI,7CI,8CI);2-(Trifluoromethoxy)benzenamine;2-Trifluoromethoxyaniline;o-(Trifluoromethoxy)aniline;
- PSA 35.25000
- LogP 2.74860
Synthetic route
-
-
64115-88-4
1-bromo-2-(trifluoromethoxy)benzene

-
-
1535-75-7
2-trifluoromethoxy aniline

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; L-2-O-methyl-chiro-inositol; copper(II) acetate monohydrate In 1-methyl-pyrrolidin-2-one at 110℃; for 12h; | 82% |
-
-
461-81-4
1-chloro-4-(trifluoromethoxy)benzene

-
A
-
1535-75-7
2-trifluoromethoxy aniline

-
B
-
1535-73-5
m-trifluoromethoxyaniline

| Conditions | Yield |
|---|---|
| Stage #1: 1-chloro-4-(trifluoromethoxy)benzene With sulfuric acid; nitric acid at 0 - 45℃; Stage #2: With palladium on activated charcoal; hydrogen; triethylamine In methanol at 80℃; under 15001.5 Torr; Temperature; Pressure; Autoclave; Overall yield = 98 percent; Overall yield = 177.12 g; |
-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
103008-51-1
2-(trifluoromethoxy)benzenesulfonyl chloride

-
-
37526-59-3
2-trifluoromethoxy-benzenesulphonic acid amide

| Conditions | Yield |
|---|---|
| 99.4% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
561304-39-0
tert-butyl N-[2-(trifluoromethoxy)phenyl]carbamate

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 2h; | 98% |
| In toluene Heating; | 76% |
-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
141-97-9
ethyl acetoacetate

-
-
1620683-57-9
3-oxo-2-(2-(2-(trifluoromethoxy)phenyl)hydrazono)butanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-trifluoromethoxy aniline With hydrogenchloride; sodium nitrite In water at 0℃; for 0.5h; Stage #2: ethyl acetoacetate With sodium acetate In ethanol; water at 0℃; | 92.3% |
| Stage #1: 2-trifluoromethoxy aniline With hydrogenchloride; sodium nitrite In water at 0℃; for 0.0833333h; Stage #2: ethyl acetoacetate With ammonium acetate In ethanol; water | |
| Stage #1: 2-trifluoromethoxy aniline With hydrogenchloride; sodium nitrite In water at 0℃; for 0.0833333h; Stage #2: ethyl acetoacetate With ammonium acetate In ethanol; water at 0℃; for 0.5h; |
-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
805237-13-2
t-butyl 2-diazopropanoate

| Conditions | Yield |
|---|---|
| With C32H30N4O4; copper(l) chloride In dichloromethane at 0℃; for 18h; Inert atmosphere; Molecular sieve; optical yield given as %ee; enantioselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With formic acid for 12h; Reflux; | 90.56% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-trifluoromethoxy aniline With hydrogenchloride; sodium nitrite In ethanol; water at 0 - 5℃; for 0.5h; Stage #2: ethyl 2-chloro-3-oxo-butyrate With sodium acetate In ethanol; water at 0 - 25℃; | 90% |

-
-
1535-75-7
2-trifluoromethoxy aniline

| Conditions | Yield |
|---|---|
| With sodium nitrite In hydrogenchloride; water | 88% |
-
-
552-16-9
ortho-nitrobenzoic acid

-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
939920-68-0
2-nitro-N-(2-(trifluoromethoxy)phenyl)benzamide

| Conditions | Yield |
|---|---|
| Stage #1: ortho-nitrobenzoic acid With thionyl chloride In N,N-dimethyl-formamide; benzene Reflux; Stage #2: 2-trifluoromethoxy aniline With triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; | 88% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1h; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromo-3-methyl-2-indolinone With nickel(II) tetrafluoroborate hexahydrate; C42H66N4O8 In ethyl acetate at 35℃; for 0.583333h; Stage #2: With N-ethyl-N,N-diisopropylamine In ethyl acetate at 0℃; for 0.166667h; Stage #3: 2-trifluoromethoxy aniline In ethyl acetate at 0℃; for 24h; enantioselective reaction; | 86% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydroxylamine hydrochloride; sodium sulfate at 55℃; Sandmeyer reaction; | 85% |
| With hydrogenchloride; hydroxylamine hydrochloride; sodium sulfate at 55℃; Sandmeyer reaction; | 85% |

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 12h; | 85% |

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 12h; Inert atmosphere; | 85% |
-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
32503-27-8
tetra(n-butyl)ammonium hydrogensulfate

| Conditions | Yield |
|---|---|
| Stage #1: 2-trifluoromethoxy aniline With chlorosulfonic acid; triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; Stage #2: With sodium hydroxide In dichloromethane; water Stage #3: tetra(n-butyl)ammonium hydrogensulfate In dichloromethane; water | 85% |

| Conditions | Yield |
|---|---|
| With 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); N,N,N′,N′-tetramethyl-N″-tert-butylguanidine; 4,4'-di-tert-butyl-2,2'-bipyridine; zinc In dimethyl sulfoxide at 80℃; for 8h; Reagent/catalyst; Inert atmosphere; | 85% |
-
-
149105-06-6
(4-bromo-3-methylphenyl)(morpholino)methanone

-
-
1535-75-7
2-trifluoromethoxy aniline

| Conditions | Yield |
|---|---|
| With potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); C37H43N2O4P In 1,4-dioxane at 90℃; for 18h; | 82% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; C29H23N6Ru2(2+)*2Cl(1-)*6H2O; toluene-4-sulfonic acid In acetonitrile at 20℃; for 5h; Irradiation; | 81% |

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; 2-trifluoromethoxy aniline Heating; Stage #2: With Eaton’s reagent Heating; | 81% |
-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
40465-45-0
p-cyanophenyl isocyanate

-
-
1416274-64-0
1-(4-cyanophenyl)-3-(2-trifluoromethoxyphenyl)urea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 80.4% |

| Conditions | Yield |
|---|---|
| With iodine; oxygen; dimethyl sulfoxide; salicylic acid at 90℃; under 760.051 Torr; for 16h; Schlenk technique; regioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate In dimethyl sulfoxide at 130℃; under 760.051 Torr; for 12h; | 78% |
-
-
1535-75-7
2-trifluoromethoxy aniline

| Conditions | Yield |
|---|---|
| In methanol for 48h; Reflux; | 77% |
-
-
4271-26-5
N-vinyl-2-oxazolidone

-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; triethylamine In acetonitrile at 20℃; for 3h; Inert atmosphere; Sealed tube; Irradiation; | 77% |


| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In dichloromethane at 20℃; for 2h; | 75% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; boron trifluoride diethyl etherate; oxygen In dimethyl sulfoxide at 105℃; for 14h; regioselective reaction; | 75% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 30 - 35℃; for 12h; | 72.3% |
-
-
56667-12-0, 108474-32-4, 56667-11-9
(Z)-2,2'-dibromostilbene

-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
1414856-44-2
5-(2-(trifluoromethoxy)phenyl)-5H-dibenzo[b,f]azepine

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; bis[2-(diphenylphosphino)phenyl] ether In toluene at 120℃; for 10h; Schlenk technique; Inert atmosphere; | 72% |
-
-
1535-75-7
2-trifluoromethoxy aniline

-
-
19182-81-1
1,4-dinitro-1H-imidazole

-
-
1297344-95-6
4-nitro-1-{2-(trifluoromethoxy)phenyl}-1H-imidazole

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; Darkness; | 71% |
2-(Trifluoromethoxy)aniline Chemical Properties
MF: C7H6F3NO
MW: 177.12
EINECS: 216-257-9
BP: 61-63 °C (15 mmHg)
Density : 1,301 g/cm3
Refractive Index : 1.4614-1.4634
FP : 54°C
BRN : 2803814
CAS DataBase Reference : 1535-75-7
NIST Chemistry Reference : 1535-75-7
Structural Formula:
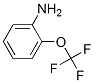
2-(Trifluoromethoxy)aniline Toxicity Data With Reference
 Xn,
Xn, T,
T, Xi
Xi Risk Statements :
R36/37/38: Irritating to eyes, respiratory system and skin
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed
R10: Flammable
2-(Trifluoromethoxy)aniline Safety Profile
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection
S26: In case of contact with eyes, rinse immediately with plenty of WATER and seek medical advice
S45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
S16: Keep away from sources of ignition - No smoking
S36: Wear suitable protective clothing
Related Products
- 2-(Trifluoromethoxy)aniline
- 15357-92-3
- 1535-91-7
- 15359-96-3
- 15359-98-5
- 15360-53-9
- 153608-51-6
- 153614-61-0
- 153-61-7
- 1536-23-8
- 15362-40-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View