-
Name
2,2'-Dichlorodiethyl ether
- EINECS 203-870-1
- CAS No. 111-44-4
- Article Data47
- CAS DataBase
- Density 1.156 g/cm3
- Solubility Slightly soluble. 1.72 g/100 mL
- Melting Point -47 °C(lit.)
- Formula C4H8Cl2O
- Boiling Point 178.7 °C at 760 mmHg
- Molecular Weight 143.013
- Flash Point 55 °C
- Transport Information UN 1916 6.1/PG 2
- Appearance colourless liquid
- Safety 27-28-36/37-45-7/9-28A
- Risk Codes 10-26/27/28-40-39/23/24/25-23/24/25-11
-
Molecular Structure
-
Hazard Symbols
 T,
T, T+,
T+, F
F
- Synonyms Ether,bis(2-chloroethyl) (8CI);1,1'-Oxybis[2-chloroethane];1,5-Dichloro-3-oxapentane;1-Chloro-2-(b-chloroethoxy)ethane;2-(2-Chloroethoxy)ethyl chloride;2-Chloroethyl ether;Bis(2-chloroethyl)ether;Bis(b-chloroethyl) ether;Chlorex;Di(b-Chloroethyl) ether;Di-2-chloroethyl ether;Diethylene glycol dichloride;Oxygenmustard;sym-Dichloroethyl ether;b,b'-Dichlorodiethyl ether;
- PSA 9.23000
- LogP 1.48060
Synthetic route

| Conditions | Yield |
|---|---|
| With 2-chloro-1,3-dimethylimidazolinium chloride; triethylamine In dichloromethane at 20℃; for 17h; Chlorination; | 92% |
| With hydrogenchloride; sulfuric acid; zinc(II) chloride at 95℃; |

| Conditions | Yield |
|---|---|
| With hexabutylguanidinium chloride at 120℃; for 4h; | 91% |

| Conditions | Yield |
|---|---|
| With thionyl chloride Reflux; | 86% |
| With thionyl chloride at 110℃; | 84% |
| With pyridine; thionyl chloride In benzene for 20h; Heating; | 35% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 30℃; for 12h; Temperature; | 81.3% |

| Conditions | Yield |
|---|---|
| With 2-chloro-ethanol |
-
-
10288-17-2
2-chloroethyl nitrite

-
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride at 165℃; |

| Conditions | Yield |
|---|---|
| With chlorine; 2-chloro-ethanol | |
| With oxirane; chlorine | |
| With oxirane; chlorine; 2-chloro-ethanol |
-
-
507-40-4
tert-butylhypochlorite

-
-
74-85-1
ethene

-
-
107-07-3
2-chloro-ethanol

-
-
111-44-4
3-oxa-1,5-dichloropentane


| Conditions | Yield |
|---|---|
| With sulfuric acid Entfernen das entstehende Wasser; | |
| With sulfuryl dichloride at 165℃; | |
| With sulfuric acid at 200 - 250℃; |
-
-
123-91-1
1,4-dioxane

-
A
-
638-56-2
1,11-dichloro-3,6,9-trioxaundecane

-
B
-
112-26-5
1,2-bis(2-chloroethoxy)ethane

-
C
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| With titanium tetrachloride 1.) 20 deg C, 2.) 160 deg C, 5 d; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
67-56-1
methanol

-
-
74-85-1
ethene

-
A
-
627-42-9
2-chloroethyl methyl ether

-
B
-
107-06-2
1,2-dichloro-ethane

-
C
-
111-44-4
3-oxa-1,5-dichloropentane

-
D
-
110-56-5
1,4-dichlorobutane

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 5 - 10℃; under 45003.6 Torr; electrolysis; | A 47 % Chromat. B 31 % Chromat. C 17 % Chromat. D 6 % Chromat. |

-
-
74-85-1
ethene

-
A
-
107-06-2
1,2-dichloro-ethane

-
B
-
111-44-4
3-oxa-1,5-dichloropentane

-
C
-
110-56-5
1,4-dichlorobutane

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; acetonitrile at 5 - 10℃; under 45003.6 Torr; electrolysis, further solvent methanol; | A 82 % Chromat. B 17 % Chromat. C 6 % Chromat. |

-
-
74-85-1
ethene

-
A
-
627-42-9
2-chloroethyl methyl ether

-
B
-
107-06-2
1,2-dichloro-ethane

-
C
-
111-44-4
3-oxa-1,5-dichloropentane

-
D
-
110-56-5
1,4-dichlorobutane

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 5 - 10℃; under 45003.6 Torr; electrolysis, further solvent aq. acetonitrile; | A 47 % Chromat. B 31 % Chromat. C 17 % Chromat. D 6 % Chromat. |

-
-
74-85-1
ethene

-
A
-
107-06-2
1,2-dichloro-ethane

-
B
-
111-44-4
3-oxa-1,5-dichloropentane

-
C
-
107-07-3
2-chloro-ethanol

| Conditions | Yield |
|---|---|
| With hypochloric acid; chlorine In various solvent(s) at 25℃; for 0.00883333h; in tributyl phosphate, in a rotor-pulsation apparatus; Title compound not separated from byproducts; | A 11.9 % Spectr. B 14.4 % Spectr. C 42.1 % Spectr. |
| With hypochloric acid; chlorine In various solvent(s) at 25℃; for 0.00883333h; in tributyl phosphate, in a rotor pulsation apparatus; Title compound not separated from byproducts; | A 11.9 % Spectr. B 14.4 % Spectr. C 42.1 % Spectr. |
| With hypochloric acid; chlorine In various solvent(s) at 25℃; for 0.00883333h; Product distribution; dependence of yield of products on time of reaction in tributyl phosphate in a rotor-pulsation apparatus; | A 11.9 % Spectr. B 14.4 % Spectr. C 42.1 % Spectr. |

-
-
7791-25-5
sulfuryl dichloride

-
-
107-07-3
2-chloro-ethanol

-
A
-
5411-48-3
sulfuric acid bis-(2-chloroethyl) ester

-
B
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| at 165℃; |

-
-
74-85-1
ethene

-
-
7782-50-5
chlorine

-
-
107-07-3
2-chloro-ethanol

-
A
-
107-06-2
1,2-dichloro-ethane

-
B
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| at 15 - 20℃; Kinetics; unter Lichtausschluss; |


| Conditions | Yield |
|---|---|
| With chlorine; sodium sulfate at 60℃; | |
| With chlorine; sodium sulfate at 60℃; |

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; polyethylene glycol chlorohydrin; chlorine; 2-chloro-ethanol at 25 - 35℃; | |
| With 1,4-dioxane; chlorine at 20 - 25℃; | |
| With polyethylene glycol mixture; chlorine |
-
-
107-07-3
2-chloro-ethanol

-
A
-
123-91-1
1,4-dioxane

-
B
-
5197-62-6
2-[2-(chloroethoxy)ethoxy]ethanol

-
C
-
628-89-7
2-(2-Chloroethoxy)ethanol

-
D
-
107-06-2
1,2-dichloro-ethane

-
E
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| at 150℃; for 5h; Product distribution; Kinetics; Rate constant; dependence of the products of ECH decomposition on temperature in sealed tube at 115, 130, 140, 150, 160 deg C; |

-
-
7791-25-5
sulfuryl dichloride

-
-
10288-17-2
2-chloroethyl nitrite

-
A
-
13891-58-2
chloroethyl chlorosulfate

-
B
-
5411-48-3
sulfuric acid bis-(2-chloroethyl) ester

-
C
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
74-85-1
ethene

-
-
7782-50-5
chlorine

-
A
-
107-06-2
1,2-dichloro-ethane

-
B
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| at 15 - 20℃; unter Lichtausschluss; |

-
-
123-91-1
1,4-dioxane

-
-
74-85-1
ethene

-
-
7782-50-5
chlorine

-
A
-
112-26-5
1,2-bis(2-chloroethoxy)ethane

-
B
-
107-06-2
1,2-dichloro-ethane

-
C
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| at 20 - 25℃; weniger als 1 Mol Chlor; | |
| at 20 - 25℃; weniger als 1 Mol Chlor; |

-
-
74-85-1
ethene

-
-
7732-18-5
water

-
-
7782-50-5
chlorine

-
A
-
107-06-2
1,2-dichloro-ethane

-
B
-
111-44-4
3-oxa-1,5-dichloropentane

-
C
-
107-07-3
2-chloro-ethanol

-
-
64-17-5
ethanol

-
-
74-85-1
ethene

-
-
7782-50-5
chlorine

-
A
-
628-34-2
2-chloroethyl ethyl ether

-
B
-
107-06-2
1,2-dichloro-ethane

-
C
-
111-44-4
3-oxa-1,5-dichloropentane

-
D
-
107-07-3
2-chloro-ethanol

-
-
646-06-0
1,3-DIOXOLANE

-
A
-
542-88-1
bis(2-chloromethyl)ether

-
B
-
1462-33-5
2-chloroethyl chloromethyl ether

-
C
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-DIOXOLANE With dichloromethane; tungsten(VI) chloride In Chloroform-D at 20℃; for 192h; Stage #2: With water In Chloroform-D at -20℃; |

-
-
3970-21-6
2-Methoxyethoxymethyl chloride

-
A
-
542-88-1
bis(2-chloromethyl)ether

-
B
-
74-87-3
methylene chloride

-
C
-
115-10-6
Dimethyl ether

-
D
-
107-30-2
chloromethyl methyl ether

-
E
-
107-06-2
1,2-dichloro-ethane

-
F
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| Stage #1: 2-Methoxyethoxymethyl chloride With dichloromethane; tungsten(VI) chloride In Chloroform-D at 20℃; for 12h; Stage #2: With water In Chloroform-D at -20℃; |

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
34270-90-1
2,2'-diiodoethyl ether

| Conditions | Yield |
|---|---|
| With sodium iodide In acetone for 120h; Heating / reflux; | 100% |
| With sodium iodide In acetone for 72h; Heating; | 81% |
| With sodium iodide In acetone for 72h; Reflux; Darkness; | 81% |
-
-
4403-78-5
ditosylethylenediamine

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
93274-33-0
N,N',N'',N'''-tetrakis(tolyl-p-sulphonyl)-1,10-dioxa-4,7,13,16-tetra-azacyclo-octadecane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide 170 deg C, 5 h; cool, 12 h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide for 5h; Heating; | 78% |
| With potassium carbonate In N,N-dimethyl-formamide at 5 - 170℃; for 30h; | 15% |
| With potassium carbonate In N,N-dimethyl-formamide at 170℃; for 12h; | 14.3% |
| With potassium carbonate In N,N-dimethyl-formamide at 170℃; for 5h; Yield given; |
-
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| With boron trichloride at -78 - 0℃; | 100% |
-
-
104-47-2
p-methoxybenzylnitrile

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
3648-78-0
4-(4-methoxyphenyl)tetrahydro-2H-pyran-4-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: p-methoxybenzylnitrile With sodium hydride In DMF (N,N-dimethyl-formamide) at 0 - 20℃; for 0.5h; Stage #2: 3-oxa-1,5-dichloropentane In DMF (N,N-dimethyl-formamide) at 0 - 20℃; for 2.33333h; | 100% |
| Stage #1: p-methoxybenzylnitrile With sodium hydroxide In N,N-dimethyl-formamide for 0.5h; Stage #2: 3-oxa-1,5-dichloropentane In N,N-dimethyl-formamide at 80 - 100℃; for 5h; | 69% |
| With sodium hydride In N,N-dimethyl-formamide; mineral oil Cooling with ice; | 64% |
| With NaH In dimethyl sulfoxide |
-
-
140-29-4
phenylacetonitrile

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
1202-81-9
4-phenyltetrahydro-2H-pyran-4-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium hydride; sodium iodide In dimethyl sulfoxide; mineral oil at 20℃; for 12h; Inert atmosphere; Sealed tube; | 99% |
| With sodium amide; toluene |
-
-
91925-82-5
3,5-dihydroxy-4-(phenylmethoxy)benzoic acid methyl ester

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
1034343-25-3
methyl 4-O-benzyl-3,5-di-O-methoxymethylgallate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 1h; Inert atmosphere; | 99% |
-
-
67-51-6
3,5-dimethyl-1H-pyrazole

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
85650-02-8
1,1'-(2,2'-oxybis(ethane-2,1-diyl))bis(3,5-dimethyl-1H-pyrazole)

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dimethyl-1H-pyrazole With potassium hydroxide In dimethyl sulfoxide at 80℃; for 1h; Stage #2: 3-oxa-1,5-dichloropentane In dimethyl sulfoxide at 80℃; Further stages.; | 98% |
-
-
16466-61-8
1,2-bis(t-butyloxycarbonyl)hydrazine

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
243973-69-5
1,4,5-oxadiazepane-4,5-dicarboxylic acid di-tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1,2-bis(t-butyloxycarbonyl)hydrazine With sodium hydride In N,N-dimethyl-formamide at -3 - 20℃; for 2.13333h; Stage #2: 3-oxa-1,5-dichloropentane In N,N-dimethyl-formamide at -3 - 20℃; Temperature; | 97.1% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 60℃; for 24h; | 97% |
-
-
104-94-9
4-methoxy-aniline

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
955095-74-6
4-(4-methoxyhenyl)morpholine nitric acid salt

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxy-aniline; 3-oxa-1,5-dichloropentane With tetrabutylammomium bromide; sodium hydroxide for 8h; Heating; Stage #2: With nitric acid at 0 - 5℃; for 12h; | 97% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dimethyl sulfoxide at 90℃; for 24h; | 96% |
-
-
135420-00-7
(4-fluoromethyl-phenyl)-acetonitrile

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
473706-10-4
4-(4-fluorophenyl)tetrahydro-2H-pyran-4-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium hydride In diethyl ether; dimethyl sulfoxide at 5 - 30℃; | 96% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 120℃; for 3h; | 96% |
| In acetonitrile at 100℃; for 48h; Inert atmosphere; | 90% |
-
-
153843-74-4
1,3-dihydroxy-4-iodo-2-nitrobenzene

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
1155368-39-0
1-iodo-2,4-bis(methoxymethoxy)-3-nitrobenzene

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; | 96% |

| Conditions | Yield |
|---|---|
| With cesium iodide In ethanol; water at 90℃; under 8250.83 Torr; Temperature; Pressure; | 95.9% |
-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
88-75-5
2-hydroxynitrobenzene

-
-
54533-65-2
1,5-bis(o-nitrophenoxy)-3-oxapentane

| Conditions | Yield |
|---|---|
| With 15-crown-5; sodium carbonate In N,N-dimethyl-formamide at 140℃; for 5h; | 95% |
| With potassium carbonate In dimethyl sulfoxide at 130℃; for 6h; | 85% |
| With potassium carbonate In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With sodium carbonate In acetonitrile for 24h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 60℃; for 24h; | 95% |
-
-
29430-29-3
2-Benzoyl-hydrazin-1-carbonsaeuremethylester

-
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl acetamide; N,N-dimethyl-formamide at 0 - 20℃; | 95% |
-
-
29366-73-2
2,4-diamino-6-(4-nitrophenyl)-1,3,5-triazine

-
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-diamino-6-(4-nitrophenyl)-1,3,5-triazine With sodium hydride In N,N-dimethyl acetamide at 55 - 60℃; for 0.166667h; Inert atmosphere; Stage #2: 3-oxa-1,5-dichloropentane In N,N-dimethyl acetamide for 4.5h; Reagent/catalyst; Temperature; Inert atmosphere; | 94.6% |
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 65℃; Thermodynamic data; Solvent; Reagent/catalyst; Concentration; Temperature; Inert atmosphere; Green chemistry; | 77% |
-
-
98-54-4
para-tert-butylphenol

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
109879-29-0
1,5-Bis(4-t-butylphenoxy)-3-oxapentane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In butan-1-ol for 18h; Heating; | 94% |
| With sodium hydroxide at 180 - 190℃; |
-
-
71676-29-4, 101833-22-1
ethyl 4,6-O-benzylidene-α-D-glucopyranoside

-
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water at 20℃; for 9h; | 94% |
-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
24345-74-2
1,5-diazido-3-oxapentane

| Conditions | Yield |
|---|---|
| With sodium azide; tetra-(n-butyl)ammonium iodide In N,N-dimethyl-formamide at 90℃; for 48h; | 93% |
| With sodium azide In N,N-dimethyl-formamide for 17h; Heating; | 62.8% |
| With sodium azide; choline chloride In N,N-dimethyl-formamide at 95℃; Rate constant; var. phase transfer catalysts; | |
| With sodium azide; cetyltributylphosphonium bromide; potassium iodide for 17h; Heating; Yield given; | |
| With sodium azide; cetylpyridinium chloride; potassium iodide In water for 20h; Heating / reflux; |
-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
109776-90-1
2,2,2-trichloro-1-fluoroethyl 2-chloroethyl ether

| Conditions | Yield |
|---|---|
| With sulfur tetrafluoride; hydrogen fluoride; chlorine at 80℃; for 5h; Product distribution; other temperature, stainless-steel autoclave, other ratio of reagents; | 93% |
| With sulfur tetrafluoride; hydrogen fluoride; chlorine at 80℃; for 5h; stainless-steel autoclave; | 93% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; Cycloaddition; | 93% |
-
-
21715-90-2
endo-N-hydroxy-5-norbornene-2,3-dicarboxyimide

-
-
111-44-4
3-oxa-1,5-dichloropentane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 2h; | 92% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 22h; Gabriel Amine Synthesis; | 64.5 g |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 24h; | 92% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 24h; Menshutkin Reaction; Heating; | 92% |
| With sodium carbonate In isopropyl alcohol at 55 - 83℃; for 8h; |
-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
41381-89-9
2-(4-phenylthiazol-2-yl)acetonitrile

-
-
1314886-95-7
4-(4-phenylthiazol-2-yl)tetrahydro-2H-pyran-4-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil Cooling with ice; | 92% |
2,2'-Dichlorodiethyl ether Consensus Reports
2,2'-Dichlorodiethyl ether Standards and Recommendations
ACGIH TLV: TWA 5 ppm; STEL 10 ppm (skin); Not Classifiable as a Human Carcinogen
DFG MAK: 10 ppm (59 mg/m3)
DOT Classification: 6.1; Label: Poison, Flammable Liquid
2,2'-Dichlorodiethyl ether Analytical Methods
2,2'-Dichlorodiethyl ether Specification
The CAS registry number of Dichloroethyl ether is 111-44-4. The IUPAC name is 1-chloro-2-(2-chloroethoxy)ethane. Its EINECS registry number is 203-870-1. In addition, the molecular formula is C4H8Cl2O and the molecular weight is 143.01. It is a kind of colourless liquid and belongs to the classes of Industrial/Fine Chemicals; Halogen compounds; A-BAlphabetic; Alpha Sort; B; BI - BZ; Volatiles/Semivolatiles.
Physical properties about this chemical are: (1)ACD/LogP: 1.19; (2)ACD/LogD (pH 5.5): 1.19; (3)ACD/LogD (pH 7.4): 1.19 ; (4)#H bond acceptors: 1; (5)#Freely Rotating Bonds: 4; (6)Polar Surface Area: Å2; (7)Index of Refraction: 1.431; (8)Molar Refractivity: 32.02 cm3; (9)Molar Volume: 123.6 cm3; (10)Polarizability: 12.69 ×10-24cm3; (11)Surface Tension: 29.3 dyne/cm; (12)Density: 1.156 g/cm3; (13)Flash Point: 55 °C; (14)Enthalpy of Vaporization: 39.8 kJ/mol; (15)Boiling Point: 178.7 °C at 760 mmHg; (16)Vapour Pressure: 1.32 mmHg at 25°C.
Preparation of Dichloroethyl ether: it can be prepared by 2,2'-oxy-bis-ethanol. This reaction will need reagent SOCl2 and pyridine and solvent benzene. The reaction time is 20 hours by heating. The yield is about 35%.
![]()
Uses of Dichloroethyl ether: it can be used as solvent of fat, oil, wax, rubber, tar, asphalt, resin and ethyl cellulose. And it is used in the preparation of coating, soil pesticides and dry lotion. In addition, it can be used in organic synthesis and gas chromatography fixed liquid. What's more, it can react with 2-amino-ethanol to get 2-morpholin-4-yl-ethanol. This reaction will need reagent Na2CO3 and solvent acetonitrile. The reaction time is 48 hours by heating. The yield is about 83%.
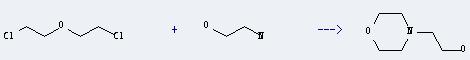
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable and has danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. There is limited evidence of a carcinogenic effect. During using it, wear suitable protective clothing and gloves. If the clothing all contaminate it, you should take off the clothing immediately. If it contact with skin, you should wash immediately with plenty of soap-suds. In case of accident or if you feel unwell, seek medical advice immediately (show label where possible). After using it, keep container tightly closed and in a well-ventilated place.
You can still convert the following datas into molecular structure:
(1)SMILES: ClCCOCCCl
(2)InChI: InChI=1/C4H8Cl2O/c5-1-3-7-4-2-6/h1-4H2
(3)InChIKey: ZNSMNVMLTJELDZ-UHFFFAOYAN
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC50 | inhalation | 500ppm/1H (500ppm) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 541, 1986. | |
| guinea pig | LD50 | skin | 300mg/kg (300mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 30, Pg. 63, 1948. | |
| mammal (species unspecified) | LD50 | oral | 112mg/kg (112mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 39(4), Pg. 86, 1974. | |
| mouse | LC50 | inhalation | 650mg/m3/2H (650mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 45, 1982. | |
| mouse | LD50 | oral | 209mg/kg (209mg/kg) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 163, 1992. |
| rabbit | LD50 | oral | 126mg/kg (126mg/kg) | "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2A, Pg. 2518, 1981. | |
| rabbit | LD50 | skin | 90mg/kg (90mg/kg) | "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2A, Pg. 2518, 1981. | |
| rat | LC50 | inhalation | 330mg/m3/4H (330mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 45, 1982. | |
| rat | LD50 | oral | 75mg/kg (75mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 30, Pg. 63, 1948. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 1114507-62-8
- 1114-51-8
- 111454-61-6
- 111-45-5
- 1114560-76-7
- 111456-85-0
- 1114-59-6
- 111465-44-2
- 111-46-6
- 111466-41-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View