-
Name
2,2'-Methylenebis(6-tert-butyl-4-methylphenol)
- EINECS 204-327-1
- CAS No. 119-47-1
- Article Data64
- CAS DataBase
- Density 1.026 g/cm3
- Solubility 7μg/L at 20℃
- Melting Point 123-127 °C(lit.)
- Formula C23H32O2
- Boiling Point 428.6 °C at 760 mmHg
- Molecular Weight 340.506
- Flash Point 181.2 °C
- Transport Information
- Appearance white to pale creamy crystalline powder
- Safety 26-36
- Risk Codes 36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms p-Cresol,2,2'-methylenebis[6-tert-butyl- (8CI);2,2'-Bis(4-methyl-6-tert-butylphenol)methane;2,2'-Methylenebis[4-methyl-6-t-butylphenol];2,2'-Methylenebis[4-methyl-6-tert-butylphenol];2,2'-Methylenebis[6-(1,1-dimethylethyl)-4-methyl)phenol;2,2'-Methylenebis[6-tert-butyl-p-cresol];Advastab 405;Agidol 2;Antage W 400;Anti Ox;Antioxidant 1;Antioxidant 2246;Antioxidant BKF;Antioxidant NG 2246;Antioxidant OMB;BKF;Baynox Plus;Bisalkofen BP;CAO 14;CAO5;Calco 2246;Catolin 14;Chemanox 21;Cyanox 2246;Lowinox 22M48;MBP 5;MDP;NG 2246;NS 6;NSC 7781;Naftonox 22M46;Noclizer NS 6;Nocrac NS 6;Nonflex MBP;Nonflex MPP;Ongrostab 2246;Plastanox 2246;Product 2246;
- PSA 40.46000
- LogP 5.90040
Synthetic route
-
-
50-00-0
formaldehyd

-
-
106-44-5
p-cresol

-
-
1634-04-4
tert-butyl methyl ether

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With sulfonated multi-walled carbon nanotubes In neat (no solvent) at 100℃; for 2.5h; Catalytic behavior; regiospecific reaction; | 100% |

-
-
109-87-5
Dimethoxymethane

-
-
2409-55-4
2-tert-Butyl-4-methylphenol

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With acid clay at 170℃; for 2h; Reagent/catalyst; Temperature; | 97% |
| With sulfuric acid at 60 - 70℃; for 2h; | 70% |
| With cation exchanger KU-2; sulfuric acid at 100℃; for 3h; Kinetics; Mechanism; Rate constant; other time, other temperature; |
-
-
50-00-0
formaldehyd

-
-
2409-55-4
2-tert-Butyl-4-methylphenol

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With sodium tetramethoxyborate In dimethyl sulfoxide at 150℃; for 0.5h; Reagent/catalyst; Temperature; Solvent; | 95% |
| In xylene at 175℃; for 10h; | 85% |
| With montmorillonite KSF In octane for 2h; Reflux; | 79% |
-
-
50-00-0
formaldehyd

-
-
96-76-4
2,4-di-tert-Butylphenol

-
A
-
14362-12-0
2,2'-methylenebis(4,6-di-tert-butylphenol)

-
B
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With montmorillonite KSF In n-heptane for 2h; Reflux; | A 85% B 77% |

-
-
50-00-0
formaldehyd

-
-
2409-55-4
2-tert-Butyl-4-methylphenol

-
A
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 1h; Condensation; acetalisation; | A 35% B 48% |

-
-
109-87-5
Dimethoxymethane

-
-
2409-55-4
2-tert-Butyl-4-methylphenol

-
A
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
B
-
4103-77-9
2-(tert-butyl)-6-(methoxymethyl)-4-methylphenol

| Conditions | Yield |
|---|---|
| With ion-exchange resin at 100℃; Product distribution; |

-
-
462-95-3
formaldehyde diethyl acetal

-
-
2409-55-4
2-tert-Butyl-4-methylphenol

-
A
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With ion-exchange resin at 100℃; Product distribution; |

-
-
2409-55-4
2-tert-Butyl-4-methylphenol

-
-
4103-77-9
2-(tert-butyl)-6-(methoxymethyl)-4-methylphenol

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With cation exchanger KU-2; sulfuric acid at 100℃; for 3h; Kinetics; Mechanism; Rate constant; other time, other temperature; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid / 60 °C 2: aq.-ethanolic HCl View Scheme |
-
-
109-87-5
Dimethoxymethane

-
-
2409-55-4
2-tert-Butyl-4-methylphenol

-
-
7664-93-9
sulfuric acid

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane


-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| at 100℃; |
-
-
27996-19-6
6-tert-butyl-2-(2'-methoxy-3'-tert-butyl-5'-methylbenzyl)-4-methylphenol

-
A
-
2219-82-1
2-tert-Butyl-6-methylphenol

-
B
-
1879-09-0
2,4-dimethyl-6-tert-butylphenol

-
C
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With zinc(II) oxide; sodium hydroxide In methanol at 220℃; for 4h; |

-
-
22204-53-1
(2S)-2-(6-methoxy(2-naphthyl))propanoic acid

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
544-76-3
Hexadecane

-
C
-
128-37-0
2,6-di-tert-butyl-4-methyl-phenol

-
D
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
E
-
2138-49-0
NSC 59850

| Conditions | Yield |
|---|---|
| With montmorillonite K10-immobilized ZnO nanocomposite pH=4.5; Catalytic behavior; Reagent/catalyst; pH-value; Concentration; Sonication; |

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
546-68-9
titanium(IV)isopropoxide

-
-
161810-86-2
bis(2,2'-methylene-bis(6-t-butyl-4-methylphenoxide))titanium

| Conditions | Yield |
|---|---|
| In diethyl ether Ar atmosphere, addn. of Ti compound to soln. of phenol at room temp., stirring (room temp., 2 h); removement of volatiles, drying (120°C); elem. anal.; | 99% |
-
-
109-99-9
tetrahydrofuran

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
860400-33-5
(cyclopentadienyl)Nb(2,2'-methylenebis(6-tert-butyl-4-methylphenoxo))(tetrahydrofuran)2

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); a soln. of ligand added slowly to a soln. of Nd complex, stirred for 1 h at 40°C; evapd. (vac.), extd. (toluene), crystd. at -10°C; elem. anal.; | 99% |

-
-
109-99-9
tetrahydrofuran

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
1076233-65-2
CpY(2,2'-methylene-bis(6-tert-butyl-4-methyl-phenoxo)(tetrahydrofuran)2

| Conditions | Yield |
|---|---|
| In tetrahydrofuran under Ar; a THF soln. of a ligand (4.50 mmol) was slowly added to a THF soln. of Y-contg. compd. (3.45 mmol); the mixt. was stirred for 6 h at 50°C; the solvent was removed under vac.; toluene was added; crystals were obtained from toluene at -5°C; elem. anal.; | 99% |


-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| In acetonitrile at 75℃; for 1.5h; | 98% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
992-92-7
titanium tetramethoxide

-
-
161810-84-0
dimethoxy[2,2'-methylenebis(6-tert-butyl-4-methylphenoxy)]titanium

| Conditions | Yield |
|---|---|
| In hexane Ar atmosphere, stirring (room temp., 3 d); filtration, washing (hexane), drying (vacuum); elem. anal.; | 97% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
39652-40-9
N,N'-bis(dichlorophosphino)aniline

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at -5 - 25℃; | 95% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
79-10-7
acrylic acid

-
-
61167-58-6
2-(2-hydroxy-3-tert-butyl-5-methylbenzyl)-4-methyl-6-tert-butylphenyl acrylate

| Conditions | Yield |
|---|---|
| With triethylamine; trichlorophosphate In nitrogen; toluene | 95% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
814-68-6
acryloyl chloride

-
-
61167-58-6
2-(2-hydroxy-3-tert-butyl-5-methylbenzyl)-4-methyl-6-tert-butylphenyl acrylate

| Conditions | Yield |
|---|---|
| With triethylamine In nitrogen; toluene | 95% |
| With triethylamine In nitrogen; toluene | 72.7% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
75-24-1
trimethylaluminum

-
-
264910-30-7
bis[Al(methyl)(2,2'-methylenebis(6-tert-butyl-4-methylphenolato))] complex

| Conditions | Yield |
|---|---|
| In hexane byproducts: CH4; under Ar, std. Schlenk or glovebox techniques; soln. of Al(CH3)3 added slowly; heat observed; mixt. cooled; ppt. filtered off; washed with cold hexane; dried in vac.; elem. anal.; | 95% |
| In hexane; toluene under Ar; 2 M soln. of AlMe3 in hexane added to soln. of bis(phenol) in toluene at room temp. (molar ratio = 1:1), mixt. stirred for 5 h; solid filtered off and washed twice with hexane; elem. anal.; | 65% |
| In hexane; toluene Ar; hexane soln. of AlMe3 added to toluene/hexane soln. of ligand (1:1 molar ratio) at -78°C, slowly warmed to room temp., stirred for 8 h under O2; ppt. filtered off, washed (hexane), elem. anal.; | 52% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| With sodium In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; | 95% |
-
-
79-41-4
poly(methacrylic acid)

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
61167-58-6
2-(2-hydroxy-3-tert-butyl-5-methylbenzyl)-4-methyl-6-tert-butylphenyl acrylate

-
-
133-59-5
Toluene-2-sulfonyl chloride

-
-
61167-60-0
2-t-butyl-6-[1-(3-t-butyl-2-hydroxy-5-methylphenyl)ethyl]-4-methylphenyl acrylate

| Conditions | Yield |
|---|---|
| With triethylamine In nitrogen; toluene | 94% |

-
-
67-56-1
methanol

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
1879-09-0
2,4-dimethyl-6-tert-butylphenol

| Conditions | Yield |
|---|---|
| With zinc(II) oxide; sodium hydroxide at 250℃; for 8h; | 94% |
-
-
109-99-9
tetrahydrofuran

-
-
67-56-1
methanol

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
860400-38-0
[(2,2'-methylenebis(6-tert-butyl-4-methylphenoxo))Nd(μ-OMe)(tetrahydrofuran)2]2

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); a soln. of 2,2'-methylenebis(6-tert-butyl-4-methylphenol) added slowly to a soln. of Nd complex at 40°C, stirred for 1 h at 40°C, methanol added, stirred overnight at room temp.; centrifuged; also isolated from the concd. soln.; elem. anal.; | 93% |

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
546-68-9
titanium(IV)isopropoxide

-
-
134754-21-5
methylenebis(6-tert-butyl-4-methylphenoxy-2-yl) diisopropoxytitanium(IV)

| Conditions | Yield |
|---|---|
| In diethyl ether Ar atmosphere, addn. of soln. of phenol to soln. of Ti compound at 0°C; concn., crystn. (-20°C), filtration; elem. anal.; | 92% |
-
-
109-99-9
tetrahydrofuran

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
1076233-63-0
CpLa(2,2'-methylene-bis(6-tert-butyl-4-methyl-phenoxo)(tetrahydrofuran)3

| Conditions | Yield |
|---|---|
| In tetrahydrofuran under Ar; a THF soln. of a ligand (4.50 mmol) was slowly added to a THF soln. of La-contg. compd. (3.00 mmol); the mixt. was stirred for 6 h at 50°C; the solvent was removed under vac.; toluene was added; crystals were obtained from toluene at -5°C; elem. anal.; | 92% |

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
7550-45-0
titanium tetrachloride

-
-
118538-56-0
methylenebis(6-tert-butyl-4-methylphenoxy-2-yl) dichlorotitanium(IV)

| Conditions | Yield |
|---|---|
| In hexane Ar atmosphere, addn. of TiCl4 to soln. of phenol, stirring (room temp., 24 h); crystn. (-20°C, 18 h), filtration, drying (vacuum); elem. anal.; | 91% |
| In hexane byproducts: HCl; TiCl4 was dropped to a soln. of MBPH2 in hexane. After 12 h standing at room temp. red crystals are formed.; | 88% |
| In hexane byproducts: HCl; under nitrogen, stirring for 12 h; filtration, washing with pentane; | 88% |
| In hexane at -40 - 20℃; for 16h; | 69% |
-
-
60-29-7
diethyl ether

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
100-99-2
triisobutylaluminum

-
-
251310-97-1
(diethyl ether)-isobutyl-(2,2'-methylene-bis(4-methyl-6-tert-butylphenolato))aluminum(III)

| Conditions | Yield |
|---|---|
| In diethyl ether; hexane dry N2-atmosphere; addn. of Al-i-Bu3 (in hexane) to 1 equiv. ligand (in Et2O) at 0°C, stirring for 2 h; evapn. (vac.), extn. into Et2O, filtration, concn., crystn. (4°C); elem. anal.; | 91% |
| In diethyl ether byproducts: isobutane; under Ar; soln. of 2,2'-methylene-bis(6-tert-butyl-4-methylphenol) added to soln. of triisobutylaluminum (5 min, room temp.); stirred (2 h); ether removed; solid recrystd. (Et2O); elem.anal.; | 63% |

-
-
14044-65-6
borane-THF

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
188707-76-8
4,8-di-tert-butyl-2,10-dimethyl-12H-dibenzo[d,g][1,3,2]dioxaborocine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: H2; (N2); after stirring in THF removal of volatiles in vacuo and heating to100°C for 90 min; crystn. (hexane); elem. anal.; | 91% |

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| In toluene under N2; soln. of ligand in toluene added to suspn. of Sm(AlMe4)2 in toluene; brought to boiling; vol. reduced; cooled to room temp.; crystd. for 24 h; elem. anal.; | 91% |
-
-
109-99-9
tetrahydrofuran

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| at 50℃; for 6h; Schlenk technique; Inert atmosphere; | 91% |

-
-
154671-38-2
bis[N,N-bistrimethylsilylamido]bis(tetrahydrofuran)ytterbium(II)

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
697278-07-2
bis(2,2'-methylenebis(6-tert-butyl-4-methylphenolate))tetrakis(tetrahydrofuran)diytterbium(II)

| Conditions | Yield |
|---|---|
| In hexane; toluene under Ar; addn. of a soln. of ytterbium complex in hexane to a soln. of ligand in toluene, stirring at room temp. for 5 h; centrifugation, ppt. washed with hexane; elem. anal.; | 90.1% |
-
-
67-56-1
methanol

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
3385-78-2
trimethyl indium

-
-
1187925-67-2
[Me6In3(OMe)(2,2'-methylenebis(4-methyl-6-tert-butylphenolate)]

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether byproducts: CH4; (N2); std. Schlenk technique; soln. of MeOH in Et2O was added to stirredsoln. of Me3In in Et2O at -76°C; warmed to room temp.; soln. of ligand in Et2O was added at -76°C; filtered; washed (Et2O); dried (vac.); elem. anal.; | 90% |

-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

| Conditions | Yield |
|---|---|
| In toluene at 20 - 100℃; Inert atmosphere; | 90% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
188707-76-8
4,8-di-tert-butyl-2,10-dimethyl-12H-dibenzo[d,g][1,3,2]dioxaborocine

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex In toluene for 18h; Reflux; | 90% |
-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
13292-87-0
dimethyl sulfide borane

-
-
188707-76-8
4,8-di-tert-butyl-2,10-dimethyl-12H-dibenzo[d,g][1,3,2]dioxaborocine

| Conditions | Yield |
|---|---|
| In toluene under N2; phenol-compd. added to B-compd. in toluene, heated at reflux for 18 h; | 90% |
-
-
112068-81-2
Sm(N(SiMe3)2)2(THF)2

-
-
119-47-1
2,2'-dihydroxy-5,5'-dimethyl-3,3'-di-tert-burtyl-1,1'-diphenylmethane

-
-
697278-06-1
bis(2,2'-methylenebis(6-tert-butyl-4-methylphenolate))hexakis(tetrahydrofuran)disamarium(II)

| Conditions | Yield |
|---|---|
| In hexane; toluene under Ar; addn. of a soln. of samarium complex in hexane to a soln. of ligand in toluene, stirring at room temp. for 5 h; centrifugation, ppt. washed with hexane; elem. anal.; | 89.6% |
2,2'-Methylenebis(6-tert-butyl-4-methylphenol) Consensus Reports
2,2'-Methylenebis(6-tert-butyl-4-methylphenol) Specification
The CAS register number of 2,2'-Methylenebis(6-tert-butyl-4-methylphenol) is 119-47-1. It also can be called as 6,6'-Di-tert-butyl-2,2'-methylenedi-p-cresol and the IUPAC name about this chemical is 2-tert-butyl-6-[(3-tert-butyl-2-hydroxy-5-methylphenyl)methyl]-4-methylphenol. The molecular formula about this chemical is C23H32O2 and the molecular weight is 340.50. It belongs to the following product categories, such as Industrial/Fine Chemicals; Organics; Diphenylmethanes (for High-Performance Polymer Research); Functional Materials; Reagent for High-Performance Polymer Research; Polymer Additives; Polymer Science; Stabilizers and so on. This chemical can be used in styrenic and olefin polymers and polyoxymethylene homo and copolymers.
Physical properties about 2,2'-Methylenebis(6-tert-butyl-4-methylphenol) are: (1)ACD/LogP: 7.03; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 7.03; (4)ACD/LogD (pH 7.4): 7.03; (5)ACD/BCF (pH 5.5): 129927.87; (6)ACD/BCF (pH 7.4): 129912.6; (7)ACD/KOC (pH 5.5): 159208.34; (8)ACD/KOC (pH 7.4): 159189.64; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 6; (12)Polar Surface Area: 18.46Å2; (13)Index of Refraction: 1.55; (14)Molar Refractivity: 105.75 cm3; (15)Molar Volume: 331.6 cm3; (16)Polarizability: 41.92x10-24cm3; (17)Surface Tension: 37.3 dyne/cm; (18)Enthalpy of Vaporization: 71.02 kJ/mol; (19)Boiling Point: 428.6 °C at 760 mmHg; (20)Vapour Pressure: 6E-08 mmHg at 25°C.
Preparation: this chemical can be prepared by formaldehyde and 2-tert-butyl-4-methyl-phenol. This reaction will need reagent KOH.
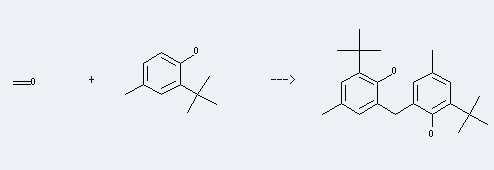
Uses of 2,2'-Methylenebis(6-tert-butyl-4-methylphenol): it can be used to produce 2-(2-Hydroxy-5-methylbenzyl)-6-tert-butyl-4-methylphenol at temperature of 25 ℃. This reaction will need reagent AlCl3 and solvent toluene with reaction time of 2 hours. The yield is about 60%.
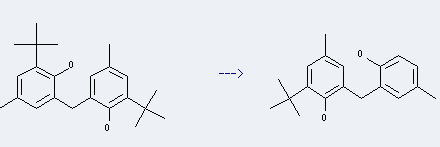
Antioxidant 264 can be produced by the reaction of P-cresol with Isobutylene, and this chemical can be used to produce the intermediate product of 2 - tert-Butyl -4-- cresol, this intermediate product can react with Formaldehyde to produce 2,2'-Methylenebis(6-tert-butyl-4-methylphenol).
.png)
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: Oc1c(cc(cc1C(C)(C)C)C)Cc2cc(cc(c2O)C(C)(C)C)C
(2)InChI: InChI=1/C23H32O2/c1-14-9-16(20(24)18(11-14)22(3,4)5)13-17-10-15(2)12-19(21(17)25)23(6,7)8/h9-12,24-25H,13H2,1-8H3
(3)InChIKey: KGRVJHAUYBGFFP-UHFFFAOYAD
(4)Std. InChI: InChI=1S/C23H32O2/c1-14-9-16(20(24)18(11-14)22(3,4)5)13-17-10-15(2)12-19(21(17)25)23(6,7)8/h9-12,24-25H,13H2,1-8H3
(5)Std. InChIKey: KGRVJHAUYBGFFP-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 11gm/kg (11000mg/kg) | Journal of Toxicological Sciences. Vol. 19, Pg. 77, 1994. | |
| rat | LDLo | oral | 10gm/kg (10000mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 38(8), Pg. 28, 1973. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 1194-73-6
- 119474-40-7
- 1194-74-7
- 119477-85-9
- 1194-78-1
- 119478-55-6
- 119478-56-7
- 119479-44-6
- 119483-47-5
- 1194-86-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View