-
Name
2,2,6,6-Tetramethylpiperidinooxy
- EINECS 219-888-8
- CAS No. 2564-83-2
- Article Data103
- CAS DataBase
- Density 1 g/cm3
- Solubility Soluble in all organic solvents. Insoluble in water.
- Melting Point 36-38 °C(lit.)
- Formula C9H18NO
- Boiling Point 193 °C
- Molecular Weight 158.264
- Flash Point 154 °F
- Transport Information UN 3263 8/PG 2
- Appearance orange crystals or powder
- Safety 26-36/37/39-45-24/25
- Risk Codes 34-36/37/38
-
Molecular Structure
-
Hazard Symbols
 C,
C, Xi
Xi
- Synonyms Piperidinooxy,2,2,6,6-tetramethyl- (7CI,8CI);2,2,6,6-Tetramethyl-1-piperidinyloxy;2,2,6,6-Tetramethylpiperidin-1-oxylradical;2,2,6,6-TetramethylpiperidineN-oxide;2,2,6,6-Tetramethylpiperidine N-oxy;2,2,6,6-Tetramethylpiperidine N-oxyl radical;2,2,6,6-Tetramethylpiperidinenitroxide;2,2,6,6-Tetramethylpiperidine oxide;2,2,6,6-Tetramethylpiperidinyl-1-oxyl;2,2,6,6-Tetramethylpiperidinyloxy;HO 6;Tanan;Tanane;Tempo;
- PSA 3.24000
- LogP 2.31290
Synthetic route

| Conditions | Yield |
|---|---|
| In 2-methyltetrahydrofuran at -135℃; Kinetics; | A n/a B 94% |

-
-
32579-76-3
1-chloro-2,2,6,6-tetramethylpiperidine

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

| Conditions | Yield |
|---|---|
| With sodium sulfate In dichloromethane Electrolysis; aq. phosphate buffer; | 82% |

-
A
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethyl-1-oxo-piperidinium; sodium acetate; acetic acid In water at 20℃; pH=4.5; UF membrane; |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In acetonitrile | |
| With dihydrogen peroxide In acetonitrile | |
| With dihydrogen peroxide In methanol; acetonitrile |
-
-
45842-10-2
2,2,6,6-tetramethyl-1-oxo-piperidinium

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

| Conditions | Yield |
|---|---|
| Alkaline conditions; |

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; Mechanism; Activation energy; Inert atmosphere; |

-
-
109-99-9
tetrahydrofuran

-
-
7031-93-8
2,2,6,6-tetramethylpiperidin-1-ol

-
-
7732-18-5
water

-
A
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

| Conditions | Yield |
|---|---|
| at -90℃; for 1h; Inert atmosphere; Schlenk technique; |

-
-
7031-93-8
2,2,6,6-tetramethylpiperidin-1-ol

-
-
5259-72-3, 10060-40-9, 111-78-4
1,5-dicyclooctadiene

-
A
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
B
-
162476-91-7
[(1,1′-bis(diphenylphosphino)ferrocene)Ni(0)(1,5-cyclooctadiene)]

-
C
-
717-74-8
1,3,5-triisopropyl benzene

| Conditions | Yield |
|---|---|
| In benzene-d6 at 20℃; for 24h; |

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
7787-70-4
copper(I) bromide

-
-
309752-65-6
6-(hydroxymethyl)-2-naphthol

-
-
78119-82-1
6-hydroxynaphthalene-2-carbaldehyde

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 99.8% |

-
-
7758-89-6
copper(I) chloride

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
309752-65-6
6-(hydroxymethyl)-2-naphthol

-
-
78119-82-1
6-hydroxynaphthalene-2-carbaldehyde

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 99.6% |

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
309752-65-6
6-(hydroxymethyl)-2-naphthol

-
-
78119-82-1
6-hydroxynaphthalene-2-carbaldehyde

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide; iron(II) chloride | 99.2% |
| In chlorobenzene | 92.6% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
502-42-1
cycloheptanone

-
-
1394206-49-5
2-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)cycloheptanone

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 99% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
93-55-0
1-phenyl-propan-1-one

-
-
1189350-76-2
1-phenyl-2-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)propan-1-one

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 99% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
121-97-1
4-Methoxypropiophenone

-
-
1394206-50-8
1-(4-methoxyphenyl)-2-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)propan-1-one

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 99% |
-
-
6285-05-8
4'-chloropropiophenone

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
1394206-51-9
1-(4-chlorophenyl)-2-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)propan-1-one

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 99% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
495-40-9
butyrophenone

-
-
1394206-52-0
1-phenyl-2-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)butan-1-one

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 99% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
611-70-1
phenyl isopropyl ketone

-
-
1394206-54-2
2-methyl-1-phenyl-2-(2,2,6,6-tetramethylpiperidin-1-yloxy)propan-1-one

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 99% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
529-34-0
3,4-dihydronaphthalene-1(2H)-one

-
-
1189350-77-3
2-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)-3,4-dihydronaphthalen-1(2H)-one

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 98% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
51315-69-6
N-tosyl-o-allylaniline

-
-
1094359-10-0
(S)-2-(2,2,6,6-tetramethyl-piperidin-1-yloxymethyl)-1-(toluene-4-sulfonyl)-2,3-dihydro-1H-indole

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate); potassium carbonate; (4R,4'R,5S,5'S)-2,2'-(propane-2,2-diyl)bis(4,5-diphenyl-4,5-dihydrooxazole) In α,α,α-trifluorotoluene at 110℃; for 24h; Inert atmosphere; optical yield given as %ee; | 97% |
| With α,α,α-trifluorotoluene; copper(II) bis(trifluoromethanesulfonate); potassium carbonate; (4R,4'R,5S,5'S)-2,2'-(propane-2,2-diyl)bis(4,5-diphenyl-4,5-dihydrooxazole) at 110℃; for 6h; enantioselective reaction; | 97% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
527737-44-6
N-(2,2-diphenyl-pent-4-enyl)-4-methylbenzenesulfonamide

-
-
1094359-32-6
1-[4,4-diphenyl-1-(toluene-4-sulfonyl)-pyrrolidin-2-ylmethoxy]-2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) bis(trifluoromethanesulfonate); potassium carbonate; (4R,4'R,5S,5'S)-2,2'-(propane-2,2-diyl)bis(4,5-diphenyl-4,5-dihydrooxazole) In α,α,α-trifluorotoluene at 120℃; under 760.051 Torr; for 24h; optical yield given as %ee; | 97% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
83-33-0
inden-1-one

-
-
1394206-53-1
2-(2,2,6,6-tetramethylpiperidin-1-yloxy)-1-indanone

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0 - 20℃; for 3h; | 97% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
100-52-7
benzaldehyde

-
-
7031-95-0
1-benzoxy-2,2,6,6-tetramethylpiperidine

| Conditions | Yield |
|---|---|
| With styrene; tert.-butylhydroperoxide; iron(II) chloride In decane; acetonitrile at 85℃; for 1h; Inert atmosphere; | 95% |
| With tert.-butylhydroperoxide; ethyl 1,5-diphenyl-4-methyl-1H-pyrazole-3-carboxylate; palladium(II) trifluoroacetate In 1,2-dichloro-ethane at 100℃; for 12h; Inert atmosphere; | 63% |
| With tert.-butylhydroperoxide; iron(III) chloride hexahydrate In water; acetonitrile Mechanism; Inert atmosphere; Reflux; | 59% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
51315-69-6
N-tosyl-o-allylaniline

-
-
1094359-65-5
C25H34N2O3S

| Conditions | Yield |
|---|---|
| With (4S,4'S)-2,2'-(1-methylethylidene)bis[4,5-dihydro-4-(p-tert-butylphenyl)] oxazole; α,α,α-trifluorotoluene; oxygen; copper(II) bis(trifluoromethanesulfonate); potassium carbonate at 110℃; under 760.051 Torr; for 6h; enantioselective reaction; | 95% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
57181-82-5
4-bromo-isobutylbenzene

-
-
1333501-16-8
2,2,6,6-tetramethyl-1-(2-methyl-1-phenylpropoxy)piperidine

| Conditions | Yield |
|---|---|
| With copper(II) trifluoroacetate; copper; 4,4'-di-tert-butyl-2,2'-bipyridine In benzene at 75℃; for 22h; Inert atmosphere; | 93% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
1416916-77-2
3,5-di-tert-butyl-N-(2,2-dimethylpent-4-en-1-yl)benzenesulfonamide

-
-
1416916-81-8
(S)-1-((1-((3,5-di-tert-butylphenyl)sulfonyl)-4,4-dimethylpyrrolidin-2-yl)methoxy)-2,2,6,6-tetramethylpiperidine

| Conditions | Yield |
|---|---|
| With α,α,α-trifluorotoluene; oxygen; copper(II) bis(trifluoromethanesulfonate); potassium carbonate; (4R,4'R)-2,2'-(propane-2,2'diyl)bis(4-phenyl-4,5-dihydrooxazole) at 20 - 110℃; under 760.051 Torr; for 6h; enantioselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite In tetrahydrofuran at 70℃; for 24h; stereoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite In tetrahydrofuran at 70℃; for 24h; Catalytic behavior; Reagent/catalyst; Temperature; Solvent; Concentration; stereoselective reaction; | 92% |
-
-
75-91-2
tert.-butylhydroperoxide

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
100-46-9
benzylamine

-
B
-
100-52-7
benzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; iodine In cyclohexane; water at 70 - 80℃; for 20h; | A n/a B 90% |


| Conditions | Yield |
|---|---|
| With tert.-butylnitrite In tetrahydrofuran at 70℃; for 24h; stereoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite In tetrahydrofuran at 70℃; for 24h; stereoselective reaction; | 90% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
12126-50-0
bis(pentamethylcyclopentadienyl)iron(II)

| Conditions | Yield |
|---|---|
| In toluene for 0.0166667h; Inert atmosphere; Schlenk technique; Glovebox; | 90% |

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
12126-50-0
bis(pentamethylcyclopentadienyl)iron(II)

-
-
1109-15-5
tris(pentafluorophenyl)borate

| Conditions | Yield |
|---|---|
| In toluene for 0.0166667h; Inert atmosphere; Schlenk technique; Glovebox; | 90% |

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
768-03-6
Phenyl vinyl ketone

-
-
1394206-65-5
3-chloro-1-phenyl-2-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)propan-1-one

| Conditions | Yield |
|---|---|
| With 2-chloro-1,3,2-benzodioxaborole In dichloromethane at 0℃; | 89% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
1356133-16-8
N-(2-allyl-phenyl)-3,5-di-tert-butylbenzenesulfonamide

-
-
1416916-79-4
(S)-1-((3,5-di-tert-butylphenyl)sulfonyl)-2-(((2,2,6,6-tetramethylpiperidin-1-yl)oxy)methyl)indoline

| Conditions | Yield |
|---|---|
| With α,α,α-trifluorotoluene; copper(II) bis(trifluoromethanesulfonate); potassium carbonate; (4R,4'R)-2,2'-(propane-2,2'diyl)bis(4-phenyl-4,5-dihydrooxazole) at 20 - 110℃; for 6h; enantioselective reaction; | 89% |
-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
-
1416916-78-3
3,5-di-tert-butyl-N-(2,2-dimethylpent-4-en-1-yl)-4-methoxybenzenesulfonamide

-
-
1416916-82-9
(S)-1-((1-((3,5-di-tert-butyl-4-methoxyphenyl)sulfonyl)-4,4-dimethylpyrrolidin-2-yl)methoxy)-2,2,6,6-tetramethylpiperidine

| Conditions | Yield |
|---|---|
| With α,α,α-trifluorotoluene; oxygen; copper(II) bis(trifluoromethanesulfonate); potassium carbonate; (4R,4'R)-2,2'-(propane-2,2'diyl)bis(4-phenyl-4,5-dihydrooxazole) at 20 - 110℃; under 760.051 Torr; for 6h; enantioselective reaction; | 88% |

| Conditions | Yield |
|---|---|
| With iridium(III) chloride; di-tert-butyl peroxide; N-methyl-N-phenylmethacrylamide at 120℃; for 24h; Mechanism; Inert atmosphere; Schlenk technique; | 88% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite In tetrahydrofuran at 70℃; for 24h; stereoselective reaction; | 88% |
2,2,6,6-Tetramethylpiperidinooxy Chemical Properties
Molecular structure of 2,2,6,6-Tetramethylpiperidinooxy (CAS NO.2564-83-2) is:
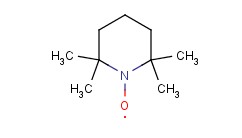
Product Name: 2,2,6,6-Tetramethylpiperidinooxy
CAS Registry Number: 2564-83-2
IUPAC Name: 1-λ1-oxidanyl-2,2,6,6-tetramethylpiperidine
Molecular Weight: 156.24532 [g/mol]
Molecular Formula: C9H18NO
XLogP3: 1.4
H-Bond Donor: 0
H-Bond Acceptor: 1
EINECS: 219-888-8
Melting Point: 36-38 °C(lit.)
Density: 1 g/cm3
Flash Point: 154 °F
Boiling Point: 193 °C
Storage temp.: 2-8°C
Other Registry Number: 125012-91-1 ;126517-51-9 ;25657-03-8 ;26933-82-4 ;54637-06-8 ;64104-42-3
Stability: Stable. Incompatible with strong acids, strong oxidizing agents. Refrigerate.
Product Categories: Analytical Chemistry;Environmentally-friendly Oxidation;ESR Spectrometry;Oxidation;Redox Catalysts (Environmentally-friendly Oxidation);Spin Labels;Synthetic Organic Chemistry
2,2,6,6-Tetramethylpiperidinooxy Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LDLo | skin | 2gm/kg (2000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0786-0607S, |
| rat | LCLo | inhalation | 4500mg/m3/2H (4500mg/m3) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0786-0607S, |
2,2,6,6-Tetramethylpiperidinooxy Safety Profile
Hazard Codes:  C;
C; Xi
Xi
Risk Statements: 34-36/37/38
R34:Causes burns.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36/37/39-45-24/25
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S24/25:Avoid contact with skin and eyes.
RIDADR: UN 3263 8/PG 2
WGK Germany: 3
RTECS: TN8991900
HazardClass: 8
PackingGroup: III
HS Code: 29333999
2,2,6,6-Tetramethylpiperidinooxy Specification
2,2,6,6-Tetramethylpiperidinooxy , its cas register number is 2564-83-2. It also can be called 2,2,6,6-Tetramethyl-1-piperidinyloxy ; 2,2,6,6-Tetramethylpiperidine-1-oxyl ; 2,2,6,6-Tetramethylpiperidinooxyl ; Tanan ; Tetramethylpiperidine nitroxide ; Tetramethylpiperidinooxy .It is a orange crystals or powder.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 256487-77-1
- 2564-95-6
- 25-65-0
- 256506-99-7
- 2565-07-3
- 256518-97-5
- 256521-65-0
- 25652-50-0
- 2565-47-1
- 25655-35-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View