-
Name
2,3-Dihydroxybenzoic acid
- EINECS 206-139-5
- CAS No. 303-38-8
- Article Data65
- CAS DataBase
- Density 1.559 g/cm3
- Solubility Soluble in water, methanol and dimethyl sulfoxide.
- Melting Point 204-206 °C(lit.)
- Formula C7H6O4
- Boiling Point 362.5 °C at 760 mmHg
- Molecular Weight 154.122
- Flash Point 187.2 °C
- Transport Information
- Appearance pale brownish to red brownish crystalline powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms o-Pyrocatechuicacid (6CI,8CI);1,2-Dihydroxybenzene-3-carboxylic acid;3-Hydroxysalicylic acid;DHBA;NSC 27435;Pyrocatechuic acid;
- PSA 77.76000
- LogP 0.79600
Synthetic route
-
-
24677-78-9
2,3-dihydroxybenzaldehyde

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile at 45℃; for 12h; chemoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With lithium hydroxide In ethanol; water at 25℃; for 2h; | 71% |
-
-
65-85-0
benzoic acid

-
A
-
303-38-8
2,3-Dihydroxybenzoic acid

-
B
-
490-79-9
2,5-dihydroxybenzoic acid.

-
C
-
99-06-9
3-Carboxyphenol

-
D
-
69-72-7
salicylic acid

-
E
-
99-96-7
4-hydroxy-benzoic acid

| Conditions | Yield |
|---|---|
| With oxygen; iron(II) sulfate In water at 40℃; for 3h; Product distribution; pH 6.8 buffer; | A 9% B 5% C 14% D 4% E 9% |

-
-
65-85-0
benzoic acid

-
A
-
303-38-8
2,3-Dihydroxybenzoic acid

-
B
-
490-79-9
2,5-dihydroxybenzoic acid.

-
C
-
99-06-9
3-Carboxyphenol

-
D
-
99-96-7
4-hydroxy-benzoic acid

| Conditions | Yield |
|---|---|
| With oxygen; iron(II) sulfate In water at 40℃; for 3h; Further byproducts given; | A 9% B 5% C 14% D 9% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide |

| Conditions | Yield |
|---|---|
| With potassium carbonate beim Schmelzen; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; chlorobenzene | |
| With hydrogenchloride at 140℃; im Rohr; | |
| With aluminium trichloride at 150℃; | |
| With hydrogen bromide at 110 - 120℃; |

| Conditions | Yield |
|---|---|
| With hydrogen iodide at 100℃; | |
| Multi-step reaction with 5 steps 1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 2 h / 30 - 35 °C 2: ammonium hydroxide / dichloromethane; water / 0.5 h / -5 - 35 °C 3: trichlorophosphate / toluene / 30 - 85 °C 4: triethylamine; aluminum (III) chloride / toluene / 9.5 h / 30 - 75 °C 5: water; hydrogenchloride / toluene / 0.5 h / 15 - 35 °C View Scheme |
-
-
62672-58-6
2-iodo-3-hydroxybenzaldehyde

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| at 100℃; |
-
-
23806-63-5
3-(benzyloxy)-2-hydroxybenzoic acid

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide at 100 - 110℃; |

| Conditions | Yield |
|---|---|
| With ammonium carbonate; water at 130 - 140℃; im geschlossenen Rohr; |

| Conditions | Yield |
|---|---|
| With potassium dicarbonate; glycerol at 180 - 210℃; | |
| With potassium dicarbonate | |
| With 2,6-dihydroxybenzoic acid decarboxylase from Rhizobium species; potassium hydrogencarbonate at 30℃; for 24h; pH=8.5; aq. phosphate buffer; Enzymatic reaction; regioselective reaction; |

| Conditions | Yield |
|---|---|
| ueber mehrere Stufen; | |
| With potassium hydroxide at 250℃; |

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
117308-63-1
Anguibactin

-
A
-
51-45-6
histamine

-
B
-
303-38-8
2,3-Dihydroxybenzoic acid

-
C
-
98135-21-8
Dehydrocystine

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; for 24h; |


| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methylene blue In ethanol at 25℃; Quantum yield; Irradiation; | |
| With dihydrogen peroxide; methylene blue In ethanol at 25℃; Irradiation; | |
| Multi-step reaction with 2 steps 1: alcohol; iodine 2: potash / beim Schmelzen View Scheme |

| Conditions | Yield |
|---|---|
| With iron(III)-acetylacetonate; dihydrogen peroxide; methylene blue at 25℃; for 4h; Product distribution; Mechanism; Irradiation; other salicylic derivatives, hydroxylation; | |
| With oxygen; potassium oxalate; iron(III) chloride In water at 25℃; Product distribution; Irradiation; role of hydrogen peroxide in dyoxygen induced hydroxylation of title compound; photochemicall and thermal (pH 7.1) hydroxylation; | |
| With dihydrogen peroxide In water at 20℃; for 0.5h; Mechanism; Product distribution; Quantum yield; Irradiation; various pH value and reaction time, HClO4 or K2CO3 presence; |

| Conditions | Yield |
|---|---|
| With Fe(III)(sal)3(3-); dihydrogen peroxide In water at 25℃; for 1h; Product distribution; Thermodynamic data; Irradiation; other times; presence of O2; |

-
-
106296-52-0
2,2-dimethylbenzo[d][1,3]dioxole-4-carboxylic acid

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In acetonitrile Irradiation; |

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With β-glucosidase for 168h; Ambient temperature; enzymatic hydrolysis; |
-
-
63-36-5, 38504-14-2, 45749-46-0
salicylate

-
A
-
303-38-8
2,3-Dihydroxybenzoic acid

-
B
-
490-79-9
2,5-dihydroxybenzoic acid.

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium carbonate In water at 20℃; for 0.5h; Mechanism; Product distribution; Quantum yield; Irradiation; various pH value and reaction time; |
-
-
69-72-7
salicylic acid

-
A
-
89-86-1
4-hydroxysalicylic acid

-
B
-
303-38-8
2,3-Dihydroxybenzoic acid

-
C
-
490-79-9
2,5-dihydroxybenzoic acid.

-
D
-
303-07-1
2,6-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iron(II) sulfate In water at 37℃; for 0.0833333h; hydroxylation; Further byproducts given. Title compound not separated from byproducts; |

-
-
69-72-7
salicylic acid

-
A
-
64-18-6
formic acid

-
B
-
617-48-1
malic acid

-
C
-
303-38-8
2,3-Dihydroxybenzoic acid

-
D
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide at 23℃; for 4h; pH=6.2; Oxidation; UV-irradiation; Further byproducts given; |

-
-
69-72-7
salicylic acid

-
A
-
1119-72-8
cis,cis-Muconic acid

-
B
-
141-82-2
malonic acid

-
C
-
303-38-8
2,3-Dihydroxybenzoic acid

-
D
-
123-31-9
hydroquinone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide at 23℃; for 2.5h; pH=6.2; Kinetics; Oxidation; UV-irradiation; |

-
-
7664-93-9
sulfuric acid

-
-
99974-86-4
3-(2,5-dimethoxy-tetrahydro-[2]furyl)-3-oxo-propionic acid methyl ester

-
A
-
2411-83-8
methyl-2,3-dihydroxybenzoate

-
B
-
303-38-8
2,3-Dihydroxybenzoic acid


| Conditions | Yield |
|---|---|
| at 160℃; |


| Conditions | Yield |
|---|---|
| at 180 - 210℃; | |
| at 180℃; |


| Conditions | Yield |
|---|---|
| at 250℃; |
-
-
7732-18-5
water

-
-
120-80-9
benzene-1,2-diol

-
A
-
99-50-3
3,4-Dihydroxybenzoic acid

-
B
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| at 140℃; |


| Conditions | Yield |
|---|---|
| sulfuric acid for 24h; Reflux; | 100% |
| With sulfuric acid | |
| With sulfuric acid |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
866528-82-7
2-hydroxy-3-(toluene-4-sulfonyloxy)-benzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 40℃; | 100% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 6h; Reflux; | 100% |
| With sulfuric acid at 0℃; for 12h; Heating / reflux; | 99% |
| With thionyl chloride at 20℃; | 99% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
72517-15-8
2,3-dihydroxy-5-bromobenzoic acid

| Conditions | Yield |
|---|---|
| With bromine; acetic acid at 20℃; for 24h; | 99% |
| With bromine In acetic acid at 20℃; for 16h; Inert atmosphere; regioselective reaction; | 99% |
| With bromine In acetic acid at 20℃; | 88% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In hydrogenchloride; methanol | 99% |
| With sodium hydrogencarbonate In hydrogenchloride; methanol | 99% |

| Conditions | Yield |
|---|---|
| sulfuric acid In diethyl ether for 12h; | 98% |
| With sulfuric acid In diethyl ether at 20℃; for 12h; Esterification; | 91% |
| With sulfuric acid for 0.416667h; Heating; | 74% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
100-39-0
benzyl bromide

-
-
95922-26-2
benzyl 2,3-bis(benzyloxy)benzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 5h; Ambient temperature; | 98% |
| With potassium carbonate In acetone for 24h; Inert atmosphere; Reflux; | 94% |
| With potassium carbonate In acetone for 24h; Reflux; Inert atmosphere; | 81% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol | 98% |
| With sulfuric acid In methanol | |
| With sulfuric acid In methanol; water | |
| Multi-step reaction with 2 steps 1: thionyl chloride / 78 °C / Inert atmosphere 2: triethylamine / 16 h / 0 - 25 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: thionyl chloride / Reflux 2: triethylamine / chloroform / 18 h / Reflux View Scheme |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
70656-95-0
chlorure de 2,3-dioxosulfinylbenzoyle

| Conditions | Yield |
|---|---|
| With thionyl chloride; urea at 40℃; for 3h; Cyclization; | 97% |
| With thionyl chloride; urea at 40℃; for 2h; Yield given; | |
| With thionyl chloride for 8h; Heating; |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 18h; Reflux; | 97% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
100-44-7
benzyl chloride

-
-
95922-26-2
benzyl 2,3-bis(benzyloxy)benzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Reflux; Inert atmosphere; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; | 91% |

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; methanesulfonic acid; iron(II) phthalocyanine; acetic acid In water; acetonitrile at 5℃; for 0.0833333h; | 96% |

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; methanesulfonic acid; iron(II) phthalocyanine; acetic acid In water; acetonitrile at 5℃; for 0.0833333h; | 96% |
-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
86748-78-9
succinimido 2,3-dihydroxybenzoate.

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In 1,4-dioxane for 16h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-Dihydroxybenzoic acid With sulfuric acid In methanol Stage #2: benzyl bromide With potassium carbonate In methanol; chloroform Stage #3: With barium(II) hydroxide In tetrahydrofuran; water at 50℃; | 95% |
| Stage #1: 2,3-Dihydroxybenzoic acid; benzyl bromide With potassium carbonate In acetone for 24h; Reflux; Stage #2: With lithium hydroxide In methanol; water for 3h; Reflux; Stage #3: With hydrogenchloride In methanol; water at 0℃; | 91% |
| With potassium hydroxide In dimethyl sulfoxide for 4h; | 89% |
-
-
110-91-8
morpholine

-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
592-87-0
lead(II) thiocyanate

-
-
1257411-46-3
8-hydroxy-2-morpholin-4-yl-4H-1,3-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-Dihydroxybenzoic acid; lead(II) thiocyanate With dibromotriphenylphosphorane In tetrahydrofuran; dichloromethane at 0 - 20℃; for 4h; Inert atmosphere; Reflux; Stage #2: morpholine In 1,4-dioxane for 4h; Reflux; | 95% |

-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
75-26-3
isopropyl bromide

-
-
85686-13-1
2,3-diisopropyloxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-Dihydroxybenzoic acid; isopropyl bromide With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 50℃; Stage #2: With barium(II) hydroxide In tetrahydrofuran; water at 50℃; | 95% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
93-97-0
benzoic acid anhydride

-
-
102184-11-2
2,3-Dibenzyloxybenzoic acid

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 16h; | 93% |

| Conditions | Yield |
|---|---|
| With sodium acetate; potassium hexacyanoferrate(III) In water at 20℃; for 0.166667h; | 92% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
824-94-2
p-methoxybenzyl chloride

-
-
1154500-34-1
4-methoxybenzyl 2,3-bis((4-methoxybenzyl)oxy)benzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone for 72h; Inert atmosphere; Reflux; | 92% |
| Stage #1: 2,3-Dihydroxybenzoic acid With tetra-(n-butyl)ammonium iodide; potassium carbonate In acetone at 25℃; for 1h; Stage #2: p-methoxybenzyl chloride In acetone Reflux; | 70% |
| With potassium carbonate; sodium iodide In N,N-dimethyl-formamide at 70℃; for 2h; | 52% |
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 2h; Inert atmosphere; | 52% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; methanesulfonic acid; iron(II) phthalocyanine; acetic acid In water; acetonitrile at 5℃; for 0.5h; | 91% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; methanesulfonic acid; iron(II) phthalocyanine; acetic acid In water; acetonitrile at 5℃; for 0.166667h; | 91% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; methanesulfonic acid; iron(II) phthalocyanine; acetic acid In water; acetonitrile at 5℃; for 0.166667h; | 91% |

| Conditions | Yield |
|---|---|
| With sodium acetate; potassium hexacyanoferrate(III) In water for 1h; Michael addition reaction; | 90% |
-
-
57260-73-8
N-BOC-1,2-diaminoethane

-
-
303-38-8
2,3-Dihydroxybenzoic acid

-
-
197636-95-6
N-(N'-tert-butyloxycarbonylethanediamino)-2,3-bis(benzyloxy)benzamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-Dihydroxybenzoic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 25℃; for 0.25h; Stage #2: N-BOC-1,2-diaminoethane In N,N-dimethyl-formamide at 25℃; for 0.5h; | 90% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 120℃; | 89.3% |

| Conditions | Yield |
|---|---|
| In toluene for 1h; Sealed tube; Heating; | 89% |

-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| In water tungstic acid, 2,3-dihydroxybenzoic acid, Me4NOH refluxed overnight in H2O; hot filtered; evapd. to dryness; washed with hot THF, CH2Cl2, Et2O; vac.dried at 70°C; | 87% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 1h; | 86% |
-
-
303-38-8
2,3-Dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With trichlorophosphate Reflux; | 86% |
2,3-Dihydroxybenzoic Acid Chemical Properties
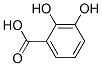
Formula Weight: 154.12
mp : 204-206 °C(lit.)
bp of 2,3-Dihydroxybenzoic Acid (303-38-8): 95-96/0.5mm
Flash Point: 187.2 °C
density of 2,3-Dihydroxybenzoic Acid (303-38-8): 1,542 g/cm3
Vapour Pressure: 6.85E-06 mmHg at 25°C
storage temp. : 2-8°C
Index of Refraction: 1.67
SOLUBILITY IN WATER: Soluble
Appearence of 2,3-Dihydroxybenzoic Acid (303-38-8): Pale Brownish to Red Brownish Crystalline Powder
2,3-Dihydroxybenzoic Acid Uses
In industrial field, 2,3-Dihydroxybenzoic Acid (303-38-8) is used as intermediate for the production of other organic chemicals, resins, polyesters, plasticizers, dyestuffs, preservatives, and rubber chemicals.
2,3-Dihydroxybenzoic Acid Toxicity Data With Reference
on the eye: Irritating effect.
on the skin: Irritant to skin and mucous membranes.
2,3-Dihydroxybenzoic Acid Safety Profile
Hazard Codes : Xi (Irritant)
Risk Statements : 36/37/38 (Irritating to eyes, respiratory system and skin)
Safety Statements : 26-36-37/39 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice; Wear suitable protective clothing; Wear suitable gloves and eye/face protection)
WGK Germany : 2
RTECS : DG8576490
Hazard Note : Irritant
2,3-Dihydroxybenzoic Acid Standards and Recommendations
LOSS ON DRYING: 0.5% max
RESIDUE ON IGNITION: 0.1% max
2,3-Dihydroxybenzoic Acid Specification
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 30339-30-1
- 3034-19-3
- 3034-22-8
- 303-42-4
- 30342-62-2
- 3034-31-9
- 3034-34-2
- 303-43-5
- 3034-38-6
- 3034-42-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View