-
Name
2,3-Dimethyl-1-butene
- EINECS 209-262-2
- CAS No. 563-78-0
- Article Data174
- CAS DataBase
- Density 0.685 g/cm3
- Solubility <0.1 g/L (20 °C )
- Melting Point -157 °C
- Formula C6H12
- Boiling Point 55.8 °C at 760 mmHg
- Molecular Weight 84.1613
- Flash Point ?1°F
- Transport Information UN 3295 3/PG 2
- Appearance Clear colourless to light yellow liquid
- Safety 16-33-62-9
- Risk Codes 11-65
-
Molecular Structure
-
Hazard Symbols
 F,
F, Xn
Xn
- Synonyms 1-Isopropyl-1-methylethylene;2,3-Dimethyl-1-butene;2-Isopropylpropene;NSC 73906;
- PSA 0.00000
- LogP 2.21850
Synthetic route
-
-
187737-37-7
propene

-
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With diethyl aluminiumcholoride; trioctyl phosphite In toluene at -10℃; under 1500.15 Torr; for 5h; Reagent/catalyst; Temperature; Inert atmosphere; Autoclave; | 90% |
| With In toluene at 70℃; for 50h; | 9% |
| With In benzene-d6 at 49℃; Rate constant; | |
| With (C6H5O)2P-N(C4H9)2; diethylaluminium chloride; Ni(β-C10H7CO2)2 In toluene at 18 - 23℃; under 750.06 Torr; Product distribution; influence of organophosphorus ligand L on the activity and selectivity of catalytic systems; | |
| With C30H38FeN3 In benzene-d6 at 23℃; for 48h; Diels-Alder Cycloaddition; |
-
-
187737-37-7
propene

-
A
-
763-29-1
2-Methyl-1-pentene

-
B
-
691-38-3
(Z)-4-methyl-2-pentene

-
C
-
691-37-2
4-Methyl-1-pentene

-
D
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With ethylaluminum dichloride; π-C3H5NiP(C6H11-cyclo)3Br In chlorobenzene at -75 - -45℃; for 0.5h; Product distribution; | A 4% B 1% C 18% D 76% |
| With VCl3((C5H3N)(C(CH3)N(C6H2)Br2Me)2) In toluene at 20℃; for 1h; | |
| VCl3((C5H3N)(C(CH3)N(C6H2)Br2Me)2) In toluene at 20℃; for 1h; | A 6 %Chromat. B 45 %Chromat. C 24 %Chromat. D 25 %Chromat. |

-
-
187737-37-7
propene

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
625-27-4
2-methyl-2-pentene

-
C
-
763-29-1
2-Methyl-1-pentene

-
D
-
4461-48-7
4-methyl-2-pentene

-
E
-
691-37-2
4-Methyl-1-pentene

-
F
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In benzene at 50℃; under 6840 Torr; for 2h; Product distribution; 0 - 60 deg C, 1 - 32 h, influence of the catalysts on the yields; | A 5.4% B 3.2% C 18.4% D 58.2% E 10.2% F 2.1% G n/a |

-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
79-29-8
2,3-dimethylbutane

-
C
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dichloride In N,N-dimethyl-formamide under 18751.5 Torr; for 0.25h; Product distribution; Ambient temperature; various time; | A 39.3% B 0.05% C 52.2% |
| With hydrogen; 1,5-hexadienerhodium(I)-chloride dimer In various solvent(s) for 2h; Ambient temperature; pH=7.6; | A 27% B 25% C 22% |
| With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; hydrogen; disodium salt of the bis(m-sulfonatophenyl)-n-butylphosphane at 60℃; under 22502.3 Torr; for 6h; Ionic liquid; chemoselective reaction; |

-
-
115982-92-8
tantalum(η5-Cp)(2,3-dimethylbutadiene){C5H6O(isopropyl)2}

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
38443-89-9
2-methyl-3-isopropyl-6-hepten-3-ol

-
C
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| Hydrolysis of starting compd.; Gas chromy.; | A 35% B 48% C 17% |


-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
A
-
38023-36-8
1,4-diphenyl-2,3-dimethylbutadiene-1,3

-
B
-
21564-72-7
1,4-Dihydro-2,3-dimethylnaphthalene

-
G
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| In benzene at 120℃; for 16h; | A n/a B n/a C 30% D n/a E 20% F 45% G n/a |

-
-
64-17-5
ethanol

-
-
125881-10-9
2,3,4,5,6-Pentamethyl-benzenesulfonic acid 1,2,2-trimethyl-3-trimethylsilanyl-propyl ester

-
A
-
558-37-2
tert-butylethylene

-
B
-
31397-75-8
(2,3-Dimethyl-but-3-enyl)-trimethyl-silane

-
E
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With water at 25℃; Rate constant; Product distribution; Mechanism; other alcohol, var. EtOH conc.; deuterium kinetic isotopic effect; | A 9% B 32% C 6% D 39% E 14% |

-
-
187737-37-7
propene

-
A
-
625-27-4
2-methyl-2-pentene

-
B
-
13269-52-8
trans-3-Hexene

-
C
-
4050-45-7
trans-2-hexene

-
D
-
691-38-3
(Z)-4-methyl-2-pentene

-
E
-
674-76-0
(E)-4-methylpent-2-ene

-
F
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With tetrabutylammonium perchlorate; bis(triphenylphosphine)nickel(II) chloride In various solvent(s) at 50℃; under 3750.3 Torr; for 24h; Product distribution; electrochemical reduction process, effect of catalyst, anode, supporting electrolyte, ligand, temperature and pressure; | A 23% B 13.5% C 4.2% D 6.1% E 32.7% F 14.8% |


| Conditions | Yield |
|---|---|
| With NaX zeolite at 300℃; | 27% |
| silver (I) ion In acetonitrile Irradiation; Yield given; | |
| With hydrogenchloride; water In decalin at 165℃; for 20h; Product distribution; in sealed tube; also with DCl/D2O, deuteration investigated; | 20.1 % Chromat. |
-
-
35913-82-7
cyclopentadienylirondicarbonyl hydride

-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
38117-54-3
cyclopentadienyl iron(II) dicarbonyl dimer

-
D
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| In pentane under N2, stirring at room temp, the react. was complete within about 1 h; evapn. (vac.), chromy. on alumina (eluent pentane for the hydrometalated products, and more polar solvents for the dimer), elem. anal., (the org. products identified by GC and NMR); | A n/a B 20% C 8% D n/a |

-
-
68258-20-8
2-(2,2-dimethylcyclopropyl)acetic acid

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
558-37-2
tert-butylethylene

-
C
-
691-37-2
4-Methyl-1-pentene

-
D
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| at 496.9℃; for 0.25h; Product distribution; Thermodynamic data; Rate constant; | A 11% B n/a C n/a D n/a E n/a |

-
-
85-44-9
phthalic anhydride

-
-
464-07-3, 20281-91-8
3,3-dimethyl-2-butanol

-
-
98-11-3
benzenesulfonic acid

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
558-37-2
tert-butylethylene

-
C
-
563-78-0
2,3-Dimethyl-1-butene


| Conditions | Yield |
|---|---|
| With zinc(II) chloride |

| Conditions | Yield |
|---|---|
| With magnesium oxide at 265℃; | |
| With lead(II) oxide at 280 - 290℃; |

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; silica gel at 300℃; bei der Destillation; |
-
-
119-64-2
tetralin

-
-
594-57-0
2-chloro-2,3-dimethylbutane

-
-
557-20-0
diethylzinc

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
560-21-4
2,3,3-Trimethyl-pentane

-
C
-
79-29-8
2,3-dimethylbutane

-
D
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| at 3℃; |


| Conditions | Yield |
|---|---|
| With aluminum(III) sulfate at 275℃; | |
| With sodium permutite at 310℃; | |
| With phosphorus pentoxide; silica gel at 300℃; |
-
-
464-07-3, 20281-91-8
3,3-dimethyl-2-butanol

-
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With oxalic acid at 110 - 120℃; | |
| With ethylmagnesium bromide at 340℃; | |
| ueber das Gleichgewicht zwischen den verschiedenen Dimethylbutenen; |
-
-
464-07-3, 20281-91-8
3,3-dimethyl-2-butanol

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With aluminum oxide at 300℃; | |
| With aluminum(III) sulfate at 275℃; | |
| With phosphoric acid at 300℃; under 128714 Torr; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic anhydride | |
| With aluminum oxide at 300℃; | |
| Multi-step reaction with 2 steps 1: 14 percent Chromat. / γ-Al2O3 / 325 °C / variation of temperature and reagent 2: 27 percent / NaX zeolite / 300 °C View Scheme | |
| Multi-step reaction with 2 steps 1: β-naphthalene-sulfonic acid View Scheme |

| Conditions | Yield |
|---|---|
| With iodine | |
| With oxalic acid at 100℃; | |
| With naphthalene-2-sulfonate |
-
-
594-52-5
2-bromo-2,3-dimethylbutane

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With triethanolamine; 2-Butoxyethanol | |
| With potassium carbonate | |
| With potassium acetate; acetic acid | |
| With potassium hydroxide |
-
-
859183-56-5
2-ethoxy-1-bromo-2,3-dimethyl-butane

-
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With propan-1-ol; zinc |
-
-
5857-68-1
di-t-butyl-1,1 ethylene

-
-
162109-20-8
1-bromo-4-naphthalenesulfonic acid

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
558-37-2
tert-butylethylene

-
C
-
563-78-0
2,3-Dimethyl-1-butene

-
D
-
115-11-7
isobutene


| Conditions | Yield |
|---|---|
| at 400℃; |
-
-
4806-33-1
2,3-dimethyl-2-butyl acetate

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| at 400℃; |
-
-
464-07-3, 20281-91-8
3,3-dimethyl-2-butanol

-
-
144-62-7
oxalic acid

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
563-78-0
2,3-Dimethyl-1-butene

-
-
594-60-5
2,3-dimethylbutan-2-ol

-
-
144-62-7
oxalic acid

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| ein Gleichgewichtsgemisch ist erhalten worden bei der Dehydratisierung; |

-
-
594-60-5
2,3-dimethylbutan-2-ol

-
-
104-15-4
toluene-4-sulfonic acid

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| ein Gleichgewichtsgemisch ist erhalten worden bei der Dehydratisierung; |


| Conditions | Yield |
|---|---|
| With sulfuric acid; α,α′-bis(2-pyridyl(tert-butyl)phosphino)-o-xylene; water; palladium(II) acetylacetonate; acetic acid at 20 - 100℃; under 30003 Torr; for 20h; Inert atmosphere; Autoclave; | 99% |
-
-
1631-73-8
trimethylstannane

-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
15095-89-3
1-Trimethylstannyl-2,3-dimethyl-butan

| Conditions | Yield |
|---|---|
| Irradiation (UV/VIS); UV-irradiation at 10°C for 80 h in a sealed tube;; | 98% |
| Irradiation (UV/VIS); UV-irradiation at 10°C for 80 h in a sealed tube;; | 98% |

| Conditions | Yield |
|---|---|
| aluminum oxide at -0.1℃; for 0.166667h; | 95% |
| x In nitromethane at 25℃; for 0.25h; | 88% |
| NiX(21) zeolite at -0.1℃; Rate constant; or ZnX(21) zeolite; |
-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
1172581-29-1
2-(2,3-dimethylbutyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With C20H22AlN2PSe In neat (no solvent) at 30℃; for 8h; Schlenk technique; Glovebox; Inert atmosphere; chemoselective reaction; | 90% |
| With chlorocarbonylbis(triphenylphosphine)rhodium(I) In dichloromethane at 20 - 40℃; for 3.5h; Inert atmosphere; | 88% |
| With C17H19Cl2CoN3O; sodium t-butanolate In tetrahydrofuran at 25℃; for 1h; Inert atmosphere; regioselective reaction; | 78 mg |
-
-
12176-06-6
hydrido(tricarbonyl)(cyclopentadienyl)molybdenum

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
563-78-0
2,3-Dimethyl-1-butene

-
B
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 react. at -75°C for 5 min; quenching by pyridine, detecting by n.m.r. spectroscopy; | A n/a B 86% |


| Conditions | Yield |
|---|---|
| With C39H60N4O4; nickel(II) tetrafluoroborate hexahydrate In 1,2-dichloro-ethane at 60℃; for 32h; optical yield given as %ee; | 86% |
-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
62127-35-9
2,3-dimethyl-1-buthyl fluoride

| Conditions | Yield |
|---|---|
| With HF-melamine; hydrogen fluoride In tetrahydrofuran at 0℃; for 1h; | 85% |
-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

-
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With C39H60N4O4; magnesium triflate In dichloromethane at 30℃; for 24h; optical yield given as %ee; enantioselective reaction; | 83% |
-
-
75-25-2
Bromoform

-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
22715-57-7
2,2,3,3-tetramethyl-1,1-dibromocyclopropane

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In pentane at 0 - 20℃; for 2.33333h; Inert atmosphere; | 82% |
-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

| Conditions | Yield |
|---|---|
| With phosgene; potassium tert-butylate In toluene at 20℃; for 12h; Inert atmosphere; | 82% |
-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
72221-03-5
2,3-dimethyl-1,2-epoxybutane

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane for 12h; Ambient temperature; | 81% |
| With sodium periodate; >*1.5H2O In dichloromethane; water at 2℃; for 15h; | 59% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 22℃; | 49% |
-
-
55962-05-5
[N-(p-tolylsulfonyl)imino]phenyliodinane

-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
956102-58-2
2-isopropyl-2-methyl-1-[(4-methyl-phenyl)sulfonyl]aziridine

| Conditions | Yield |
|---|---|
| With Cu(C6H5NC6H2(C(CH3)3)2O)2 In acetonitrile at 20℃; for 5h; Schlenk technique; Inert atmosphere; | 81% |
-
-
99-87-6
4-methylisopropylbenzene

-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
2084-69-7
1,1,2,4,4,7-hexamethyl-1,2,3,4-tetrahydro-naphthalene

| Conditions | Yield |
|---|---|
| Stage #1: 4-methylisopropylbenzene; 2,3-Dimethyl-1-butene With isobutyryl chloride for 0.5h; Stage #2: With antimony(III) chloride In dichloromethane at 25℃; for 2h; Reagent/catalyst; Solvent; Temperature; | 80.24% |
| With aluminium trichloride; tertiary butyl chloride; Aliquat 336 In cyclohexane at 20℃; | 47.5% |
| With aluminum (III) chloride; tertiary butyl chloride In dichloromethane; cyclohexane at 10℃; for 0.416667h; Temperature; Solvent; Reagent/catalyst; | 44.22% |
| With ion-exchange resin + form> | |
| With aluminum (III) chloride; tertiary butyl chloride In cyclohexane |
-
-
102690-46-0
phenyl buta-2,3-dienoate

-
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With ethylaluminum dichloride In hexane; dichloromethane at 20℃; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; water In acetonitrile at 20℃; for 48h; Irradiation; Inert atmosphere; Sealed tube; | 75% |
-
-
16312-79-1
4-methylurazole

-
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| In ethyl acetate Ambient temperature; | A 73% B 15% |
| In ethyl acetate for 12h; Ambient temperature; | A 73% B 15% |

-
-
50-00-0
formaldehyd

-
-
563-78-0
2,3-Dimethyl-1-butene

-
A
-
74126-47-9
3,4-dimethylpent-3-en-1-ol

-
B
-
76019-22-2
3-methylene-4-methylpentan-1-ol

| Conditions | Yield |
|---|---|
| With dimethylaluminum chloride In dichloromethane at 25℃; for 12h; | A 24% B 72% |


| Conditions | Yield |
|---|---|
| Stage #1: 2,3-Dimethyl-1-butene With n-butyllithium; potassium tert-butylate In tetrahydrofuran at -78 - -45℃; for 1.08333h; Stage #2: epichlorohydrin In tetrahydrofuran at -78℃; for 0.5h; | 72% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 8h; Heating; | 71% |
-
-
109976-00-3
1,2,3-tri-tert-butylazadiboridine

-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
367941-22-8
1,2,5,-tri-tert-butyl-3-isopropyl-3-methyl-1-aza-2,5-diboracyclopentane

| Conditions | Yield |
|---|---|
| In pentane soln. of compds. stirred at room temp. for 3 h; volatiles removed in vac., pure liquid product condensed into cooled receiver at 60°C/0.001 mbar; elem. anal.; | 71% |
-
-
872513-93-4
(2S)-methyl 2-N-benzoylamino-5-methylhex-4-enoate

-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
1440507-70-9
(S,E)-methyl 2-benzamido-5,6-dimethylhept-4-enoate

| Conditions | Yield |
|---|---|
| With Hoveyda-Grubbs catalyst second generation In benzene at 100℃; for 24h; Inert atmosphere; Sealed tube; | 71% |
-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
228861-07-2
(22R,5'ξ)-22-acetoxy-22-(5'-isopropyl-5'-methylisoxazolin-3'-yl)-6,6-ethylenedioxy-3α,5-cyclo-23,24-dinorcholane

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; triethylamine In chloroform for 2h; | 70% |

| Conditions | Yield |
|---|---|
| With 2,4,6-Triisopropylthiophenol; C36H16F16IrN4(1+)*F6P(1-) In 1,4-dioxane at 35℃; Glovebox; Irradiation; chemoselective reaction; | 70% |

| Conditions | Yield |
|---|---|
| With acetylacetonatodicarbonylrhodium(l); C50H59Fe2NP2; hydrogen In toluene at 120℃; under 56255.6 Torr; for 16h; | 69% |
| With hydrogen; carbonylhydridetris(triphenylphosphine)rhodium(I); (-)-Diop at 95℃; under 40 Torr; for 96h; Yield given; | |
| With carbonylhydridetris(triphenylphosphine)rhodium(I); hydrogen In toluene at 60℃; under 11400 Torr; for 2h; Product distribution; Further Variations:; Solvents; Reagents; Pressures; |

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate In chloroform for 24h; | 68% |
-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
684-16-2
Hexafluoroacetone

-
-
16203-14-8
1,1,1-trifluoro-4,5-dimethyl-2-trifluoromethyl-hex-4-en-2-ol

| Conditions | Yield |
|---|---|
| With 3 Å molecular sieves at 100℃; for 0.166667h; Microwave irradiation; | 67% |

-
-
563-78-0
2,3-Dimethyl-1-butene

-
-
1356386-29-2
C2B10H11CH2CH(CH3)CH(CH3)2

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: thallium(I) chloride; (Ar); using Schlenk techniques; addn. of soln. of HBCl2*dioxane in CH2Cl2 to stirred soln. of 2,2-dimethyl-1-butene in CH2Cl2 for 10 min, stirring at room temp. for 2 h, addn. to suspn. of Tl2C2B9H11 in CH2Cl2 for 5 min, stirring at room temp. for 18 h; filtration through paper filter, washing of ppt. on filter with CH2Cl2, evapn. with silica, placing on top of silica column in hexane, eluting with hexane, evapn., drying under vac.; | 66% |


| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In hexane at -30 - 20℃; for 0.166667h; Ene Reaction; Inert atmosphere; regioselective reaction; | A 31% B 63% |

2,3-Dimethyl-1-butene Chemical Properties
Chemical Name: 2,3-Dimethyl-1-butene
IUPAC NAME: 2,3-Dimethyl-1-butene
CAS No.: 563-78-0
EINECS: 209-262-2
EC Class: highly flammable
Molecular Formula: C6H12
Molecular Weight: 84.16 g/mol
Melting Point: -157 °C
Density: 0.685 g/cm3
Boiling Point: 55.8 °C at 760 mmHg
Following is the structure of 2,3-Dimethyl-1-butene (563-78-0):
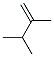
Product Categories about 2,3-Dimethyl-1-butene (563-78-0) are Acyclic ; Alkenes ; Organic Building Blocks
The chemical synonymous of 2,3-Dimethyl-1-butene (563-78-0) are (CH3)2CHC(CH3)=CH2 ; 2,3-Dimethylbutene-1 ; 1-Methyl-1-isopropylethylene ; BISOMER DMB 2,3-Dimethyl-1-butene ; Dimethylbutene
2,3-Dimethyl-1-butene Safety Profile
Hazard Codes:
F: Flammable ![]()
Xn: Harmful ![]()
Risk Statements about 2,3-Dimethyl-1-butene (563-78-0):
R11 Highly flammable.
R36/37/38 Irritating to eyes, respiratory system and skin.
R65 Harmful: may cause lung damage if swallowed.
Safety Statements about 2,3-Dimethyl-1-butene (563-78-0):
S16 Keep away from sources of ignition - No smoking.
S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39 Wear suitable gloves and eye/face protection.
S62 If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label.
2,3-Dimethyl-1-butene Specification
1. Storage: Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
2. Handling: Wash thoroughly after handling. Use only in a well ventilated area. Ground and bond containers when transferring material. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Take precautionary measures against static discharges. Avoid contact with heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
3. Personal Protection: Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure.
4. Fire Fighting: Wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Use water spray to keep fire-exposed containers cool. Extremely flammable liquid. Containers may explode in the heat of a fire. Will be easily ignited by heat, sparks or flame. Extinguishing media: Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water. In case of fire use water spray, dry chemical, carbon dioxide, or appropriate foam.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 56378-59-7
- 56378-72-4
- 563-79-1
- 5637-94-5
- 56379-89-6
- 563-80-4
- 5638-06-2
- 5638-12-0
- 56-38-2
- 56382-90-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View