-
Name
2,4,5-TRIMETHYLANILINE
- EINECS 205-282-0
- CAS No. 137-17-7
- Article Data17
- CAS DataBase
- Density 0.78
- Solubility AUTOIGNITION
- Melting Point 62 - 65 C
- Formula C9H13 N
- Boiling Point 234 - 235 C
- Molecular Weight 135.209
- Flash Point -5 ºC
- Transport Information UN 1648
- Appearance Colorless to reddish yellow liquid
- Safety 2352436
- Risk Codes R11;R20/21/22;R36
-
Molecular Structure
-
Hazard Symbols


- Synonyms Aniline,2,4,5-trimethyl- (6CI,7CI,8CI); 2,4,5-Trimethylaniline;2,4,5-Trimethylbenzenamine; 2,4,5-Trimethylphenylamine; NSC 37004; NSC 5297;Pseudocumidine; y-Cumidine
- PSA 26.02000
- LogP 2.77520
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal under 22800 Torr; Ambient temperature; | 97% |
| With sodium dithionite | |
| With iron | |
| palladium In methanol | |
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 35℃; for 2h; |

| Conditions | Yield |
|---|---|
| at 235℃; |
-
-
67-56-1
methanol

-
-
638-03-9
m-toluidine hydrochloride

-
A
-
488-71-1
2,3,4,6-tetramethylaniline

-
B
-
3238-38-8
isodurenol

-
C
-
137-17-7
2,4,5-trimethylaniline

-
D
-
95-64-7
4-amino-o-xylene

| Conditions | Yield |
|---|---|
| je nach Mengenverhaeltnis und Temperatur; Produkt 5:Pentamethylphenol; |


| Conditions | Yield |
|---|---|
| at 300 - 320℃; |
-
-
121-72-2
N,N-dimethyl-m-toluidine

-
-
5435-44-9, 22243-66-9
(E)-3-Ureido-but-2-enoic acid ethyl ester

-
-
137-17-7
2,4,5-trimethylaniline

| Conditions | Yield |
|---|---|
| at 300℃; |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 300℃; |
-
-
64-17-5
ethanol

-
-
108-24-7
acetic anhydride

-
-
64-19-7
acetic acid

-
-
94378-05-9
1-(2,4,5-Trimethyl-phenylazo)-[2]naphthol

-
A
-
137-17-7
2,4,5-trimethylaniline

-
B
-
88166-77-2
2,4,5-trimethylacetanilide

-
C
-
117-93-1
1-acetylamino-naphthalen-2-ol

-
D
-
2834-92-6
1-amino-2-naphthol

-
-
7647-01-0
hydrogenchloride

-
-
67-56-1
methanol

-
-
95-78-3
2,5-Dimethylaniline

-
-
137-17-7
2,4,5-trimethylaniline

| Conditions | Yield |
|---|---|
| at 300 - 320℃; |

-
-
7647-01-0
hydrogenchloride

-
-
50-00-0
formaldehyd

-
-
95-78-3
2,5-Dimethylaniline

-
A
-
137-17-7
2,4,5-trimethylaniline

-
B
-
5339-30-0
bis-(4-amino-2,5-dimethyl-phenyl)-methane

| Conditions | Yield |
|---|---|
| Erhitzen des Reaktionsprodukts mit Calciumhydroxid; | |
| Erhitzen des Reaktionsprodukts mit Natriumcarbonat; |


-
-
137-17-7
2,4,5-trimethylaniline

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin |

| Conditions | Yield |
|---|---|
| at 300 - 320℃; |

| Conditions | Yield |
|---|---|
| at 300 - 320℃; |

-
-
137-17-7
2,4,5-trimethylaniline

| Conditions | Yield |
|---|---|
| Darstellung; |


| Conditions | Yield |
|---|---|
| in verschiedenen Loesungsmitteln; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 38 percent / HNO3 (d= 1,40), H2SO4 (d = 1,83) 2: 97 percent / H2, aq. HCl / 10percent Pd/C / 22800 Torr / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: nitric acid; sulfuric acid 2: Na2S2O4 View Scheme |

| Conditions | Yield |
|---|---|
| 90% | |
| With sulfuric acid |
-
-
2033-24-1
cycl-isopropylidene malonate

-
-
137-17-7
2,4,5-trimethylaniline

-
-
149-73-5
trimethyl orthoformate

-
-
1129400-01-6
2,2-dimethyl-5-{[(2,4,5-trimethylphenyl)amino]methylene}-1,3-dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; trimethyl orthoformate for 2h; Inert atmosphere; Reflux; Stage #2: 2,4,5-trimethylaniline for 2h; Inert atmosphere; Reflux; | 82% |


| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; Cooling; | 79% |
-
-
50-00-0
formaldehyd

-
-
137-17-7
2,4,5-trimethylaniline

-
-
111437-12-8
1,2,4,7,8,10-hexamethyl-6H,12H-5,11-methanodibenzo<1,5>diazocine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 24h; Ambient temperature; | 78% |
-
-
475158-80-6
(R)-3,5-dichloro-1-(1-cyclopropylpropyl)pyrazin-2(1H)-one

-
-
137-17-7
2,4,5-trimethylaniline

-
-
1173523-71-1
(R)-5-chloro-1-(1-cyclopropylpropyl)-3-(2,4,5-trimethylphenylamino)pyrazin-2(1H)-one

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene for 5h; Inert atmosphere; Reflux; | 71% |
-
-
137-17-7
2,4,5-trimethylaniline

-
-
532-55-8
Benzoyl isothiocyanate

-
-
117174-81-9
1-Benzoyl-3-(2,4,5-trimethyl-phenyl)-thiourea

| Conditions | Yield |
|---|---|
| In acetone for 0.5h; Heating; | 70% |

| Conditions | Yield |
|---|---|
| With C18H15IMnN3O3; sodium t-butanolate In toluene at 120℃; for 18h; Inert atmosphere; Schlenk technique; | 56% |
-
-
1173435-13-6
(R)-3,5-dichloro-1-(1-cyclopropylethyl)pyrazin-2(1H)-one

-
-
137-17-7
2,4,5-trimethylaniline

-
-
1173435-75-0
(R)-5-chloro-1-(1-cyclopropylethyl)-3-(2,4,5-trimethylphenylamino)pyrazin-2(1H)-one

| Conditions | Yield |
|---|---|
| Stage #1: (R)-3,5-dichloro-1-(1-cyclopropylethyl)pyrazin-2(1H)-one; 2,4,5-trimethylaniline With sodium hexamethyldisilazane In tetrahydrofuran at 0 - 10℃; Inert atmosphere; Stage #2: With water; sodium hydrogencarbonate In tetrahydrofuran | 41% |
-
-
204394-69-4
4-chloro-2-(3-pyridyl)-6-(trifluoromethyl)pyrimidine

-
-
137-17-7
2,4,5-trimethylaniline

-
-
438249-55-9
4-(2,4,5-Trimethylanilino)-2-(3-pyridinyl)-6-(trifluoromethyl)pyrimidine

| Conditions | Yield |
|---|---|
| 30% |
-
-
137-17-7
2,4,5-trimethylaniline

-
-
4891-38-7
phenylpropynoic acid methyl ester

-
A
-
1041644-10-3
(E)-methyl 3-(2-amino-3,5,6-trimethylphenyl)-3-phenylpropenoate

-
B
-
1041644-09-0
(E)-methyl 3-(3-amino-2,5,6-trimethylphenyl)-3-phenylpropenoate

| Conditions | Yield |
|---|---|
| With fluorosulphonic acid at -30℃; for 1h; | A 18% B 30% |
| With fluorosulphonic acid at -30℃; | A 18% B 30% |

-
-
137-17-7
2,4,5-trimethylaniline

-
-
30824-26-1
N,N-bis(2-Chloroethyl)-3-methoxypropylamine Hydrochloride

-
-
76088-51-2
1-(3-Methoxypropyl)-4-(2,4,5-trimethylphenyl)piperazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-ethyl-N,N-diisopropylamine; benzenesulfonyl chloride 1) ethanol, reflux, 5.5 h; 2) tetrahydrofurane, reflux, 15 min;; | 28% |
-
-
137-17-7
2,4,5-trimethylaniline

-
-
7515-16-4
methyl 3-(4-methylphenyl)prop-2-ynoate

-
A
-
1139721-55-3
(E)-methyl 3-(2-amino-3,5,6-trimethylphenyl)-3-(4-methylphenyl)acrylate

-
B
-
1139719-59-7
(E)-methyl 3-(3-amino-2,5,6-trimethylphenyl)-3-(4-methylphenyl)acrylate

| Conditions | Yield |
|---|---|
| With fluorosulphonic acid at -75℃; | A 19% B 11% |

-
-
7515-17-5
methyl 3-(4-methoxyphenyl)propynoate

-
-
137-17-7
2,4,5-trimethylaniline

-
B
-
1139719-60-0
(E)-methyl 3-(3-amino-2,5,6-trimethylphenyl)-3-(4-methoxyphenyl)acrylate

| Conditions | Yield |
|---|---|
| With fluorosulphonic acid at -75℃; | A 9% B 17% |

-
-
137-17-7
2,4,5-trimethylaniline

-
-
80221-02-9
4-(p-tolyl)but-3-yn-2-one

-
-
1139719-64-4
(Z)-4-(3-amino-2,5,6-trimethylphenyl)-4-(4-methylphenyl)but-3-en-2-one

| Conditions | Yield |
|---|---|
| With fluorosulphonic acid at -30℃; | 16% |
-
-
553-90-2
Dimethyl oxalate

-
-
137-17-7
2,4,5-trimethylaniline

-
B
-
778595-30-5
N,N'-bis-(2,4,5-trimethyl-phenyl)-oxalamide


| Conditions | Yield |
|---|---|
| With hydrogenchloride at 252℃; |
-
-
67-56-1
methanol

-
-
137-17-7
2,4,5-trimethylaniline

-
A
-
488-71-1
2,3,4,6-tetramethylaniline

-
B
-
40505-10-0
1,2,4,5,7,8-hexamethyl-acridine

| Conditions | Yield |
|---|---|
| at 252℃; im Autoklaven; |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide Behandeln des Reaktionsprodukts mit Chloressigester in Alkohol und Kochen mit Anisaldehyd in Eisessig; |
-
-
85-44-9
phthalic anhydride

-
-
137-17-7
2,4,5-trimethylaniline

-
-
40101-46-0
N-(2,4,5-trimethyl-phenyl)-phthalimide

| Conditions | Yield |
|---|---|
| man spaltet das hierbei entstandene N-<2.4.5-Trimethyl-phenyl>-phthalimid durch 1/2-stdg. Kochen mit alkoh. Kali; |

| Conditions | Yield |
|---|---|
| und Eingiessen der Fluessigkeit in Kalilauge; |

-
-
50-00-0
formaldehyd

-
-
17579-79-2
2-methyl-6-hydroxynaphthalene

-
-
137-17-7
2,4,5-trimethylaniline

-
-
111584-57-7
3,8,10,11-tetramethyl-benz[a]acridine

2,4,5-Trimethylaniline Chemical Properties
Molecular Structure of 2,4,5-Trimethylaniline (CAS NO.137-17-7):
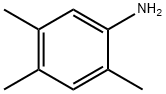
EINECS: 205-282-0
IUPAC Name: 2,4,5-Trimethylaniline
Molecular Formula: C9H13N
Molecular Weight: 135.206220 g/mol
XLogP3-AA: 2.3
H-Bond Donor: 1
H-Bond Acceptor: 1
Canonical SMILES: CC1=CC(=C(C=C1C)N)C
InChI: InChI=1S/C9H13N/c1-6-4-8(3)9(10)5-7(6)2/h4-5H,10H2,1-3H3
InChIKey: BMIPMKQAAJKBKP-UHFFFAOYSA-N
Index of Refraction: 1.552
Molar Refractivity: 44.96 cm3
Molar Volume: 140.5 cm3
Surface Tension: 36.7 dyne/cm
Density: 0.962 g/cm3
Flash Point: 102.9 °C
Enthalpy of Vaporization: 47.12 kJ/mol
Boiling Point: 234.5 °C at 760 mmHg
Vapour Pressure: 0.0527 mmHg at 25 °C
Melting Point: 62 °C
Water Solubility: 1009 mg/L at 25 °C
Storage Temp.: 2-8 °C
2,4,5-Trimethylaniline Toxicity Data With Reference
| 1. | mma-sat 10 µg/plate | ENMUDM Environmental Mutagenesis. 8 (Suppl 7)(1986),1. | ||
| 2. | dnd-ham:lng 10 mmol/L | MUREAV Mutation Research. 77 (1980),317. | ||
| 3. | orl-rat LD50:1250 mg/kg | MarJV# Personal Communication from Josef V. Marhold, VUOS, 539-18, Pardubice, Czechoslovakia, to the Editor of RTECS, Cincinnati, OH, March 29, 1977 29MAR77 . |
2,4,5-Trimethylaniline Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 27 ,1982,p. 177.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . NCI Carcinogenesis Bioassay (feed); Clear Evidence: mouse, rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-160 ,1979. .
2,4,5-Trimethylaniline Safety Profile
Safety Information of 2,4,5-Trimethylaniline (CAS NO.137-17-7):
Hazard Codes: F  ,Xn
,Xn  ,N
,N  ,T
,T 
Hazard Note: Harmful
Risk Statements: 11-20/21/22-36-51/53-23/24/25-45
R11:Highly flammable
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed
R36:Irritating to eyes
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed
R45:May cause cancer
Safety Statements: 16-36/37-61-45-53
S16:Keep away from sources of ignition
S36/37:Wear suitable protective clothing and gloves
S61:Avoid release to the environment. Refer to special instructions / safety data sheets
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S53:Avoid exposure - obtain special instructions before use
RIDADR: UN1648 3/PG 2
RTECS: BZ0520000
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. Used as a dye, pigment, and printing ink. See also ANILINE DYES.
2,4,5-Trimethylaniline Standards and Recommendations
DFG MAK: Animal Carcinogen, Suspected Human Carcinogen
2,4,5-Trimethylaniline Specification
2,4,5-Trimethylaniline with CAS Registry Number of 137-17-7 is white crystals or beige powder. It is also called for 1,2,4-Trimethyl-5-aminobenzene ; 1-Amino-2,4,5-trimethylbenzene ; 2,4,5-Trimethylanilin ; 2,4,5-Trimethylanilin [Czech] ; 2,4,5-Trimethylbenzenamine ; Benzenamine, 2,4,5-trimethyl- ; CCRIS 608 ; HSDB 2908 ; NCI-C02299 ; NSC 37004 ; Pseudocumidine ; Pseudokumidin ; Pseudokumidin [Czech] ; Psi-cumidine ; psi-Cumidine ; Aniline, 2,4,5-trimethyl- ; Benzenamine, 2,4,5-trimethyl- (9CI) . 2,4,5-Trimethylaniline may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 137178-88-2
- 137181-56-7
- 13718-22-4
- 137182-37-7
- 13718-26-8
- 13718-47-3
- 13718-50-8
- 13718-55-3
- 13718-58-6
- 13718-59-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View