-
Name
2,4-Dichloropyrimidine
- EINECS 223-508-6
- CAS No. 3934-20-1
- Article Data45
- CAS DataBase
- Density 1.493 g/cm3
- Solubility Soluble in water (partly), methanol, chloroform, and ethyl acetate.
- Melting Point 57-61 °C(lit.)
- Formula C4H2Cl2N2
- Boiling Point 209.1 °C at 760 mmHg
- Molecular Weight 148.979
- Flash Point 104.3 °C
- Transport Information UN 1759
- Appearance White solid
- Safety 26-36-28A
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,6-Dichloropyrimidine;Pyrimidine, 2,4-dichloro-;
- PSA 25.78000
- LogP 1.78340
Synthetic route

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 105℃; | 98% |
| With phosgene; Triphenylphosphine oxide In nitrobenzene at -5 - 105℃; for 0.666667h; Inert atmosphere; | 90% |
| With N-ethyl-N,N-diisopropylamine; trichlorophosphate at 0 - 95℃; for 3h; Sealed tube; | 82% |

| Conditions | Yield |
|---|---|
| With dmap; thionyl chloride; bis(trichloromethyl) carbonate at 65 - 70℃; | 95% |
| With dmap; thionyl chloride; bis(trichloromethyl) carbonate for 9.5h; Reflux; Green chemistry; | 95% |
| With pyridine; trichlorophosphate at 180℃; under 150.015 - 900.09 Torr; for 2h; Neat (no solvent); | 91% |
| With trichlorophosphate | |
| With thionyl chloride at 20℃; |
-
-
49844-90-8
4-Chloro-2-methylthiopyrimidine

-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In dichloromethane; acetonitrile at 0℃; for 24h; | 60% |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride |

| Conditions | Yield |
|---|---|
| at 200℃; |

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In water; acetone |
-
-
141-90-2
2-thiouracil

-
-
10026-13-8, 874483-75-7
phosphorus pentachloride

-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| at 140℃; |
-
-
1336-21-6
ammonium hydroxide

-
-
166752-02-9
4,6-dichloro-5-methoxy-2-(2-naphthylmethylthio)-pyrimidine

-
A
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| In acetonitrile | |
| In acetonitrile |

-
-
138918-23-7
4,6-dihydroxy-5-benzylthiopyrimidine

-
A
-
3934-20-1
2,6-Dichloropyrimidine

-
B
-
138918-24-8
4,6-dichloro-5-benzylthiopyrimidine

| Conditions | Yield |
|---|---|
| With trichlorophosphate In chloroform | |
| With trichlorophosphate In chloroform |
-
-
3724-43-4, 149409-22-3
Vilsmeier reagent

-
-
109-77-3
malononitrile

-
A
-
3934-20-1
2,6-Dichloropyrimidine

-
B
1-(dimethylamine)-2,2-dicyanoethylene -
16849-88-0
1-(dimethylamine)-2,2-dicyanoethylene

| Conditions | Yield |
|---|---|
| Stage #1: Vilsmeier reagent; malononitrile at -10℃; Stage #2: With sodium hydroxide In water pH=3; Cooling with ice; |

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
66-22-8
uracil

-
A
-
3934-20-1
2,6-Dichloropyrimidine

-
B
-
871254-61-4
2,4-dichloro-5-pyrimidinecarboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at -5 - 5℃; for 0.5h; Stage #2: uracil at 5℃; for 2h; Temperature; |

-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
940875-66-1
1-(2-hydroxy-3-methyl-5-fluorobenzyl)-3-(3-t-butyl-1-p-tolyl-1H-pyrazol-5-yl)urea

-
-
940871-30-7
1-(2-(2-chloropyrimidin-4-yloxy)-3-methyl-5-fluorobenzyl)-3-(3-t-butyl-1-p-tolyl-1H-pyrazol-5-yl)urea

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 0 - 20℃; for 24h; | 100% |
| With sodium hydroxide In water; acetone at 20℃; |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
5188-07-8
sodium thiomethoxide

-
-
5909-26-2
2,4-Bis(methylthio)pyrimidine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 80℃; for 4h; | 100% |
-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In water; acetonitrile at 80℃; for 2.5h; Inert atmosphere; | 100% |
-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In water; acetonitrile at 80℃; for 2.5h; Inert atmosphere; | 100% |
-
-
3934-20-1
2,6-Dichloropyrimidine


| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In water; acetonitrile at 80℃; for 2h; Inert atmosphere; | 100% |
-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In water; acetonitrile at 80℃; for 2.5h; Inert atmosphere; | 100% |
-
-
3934-20-1
2,6-Dichloropyrimidine


| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In water; acetonitrile at 80℃; for 2.5h; Inert atmosphere; | 100% |
-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-2-(difluoromethyl)-4-fluoro-1-isopropyl-1H-benzo[d]imidazole With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; bis(pinacol)diborane In 1,4-dioxane at 100℃; Inert atmosphere; Stage #2: 2,6-Dichloropyrimidine With potassium carbonate In 1,4-dioxane; water at 80℃; for 2h; Inert atmosphere; | 99.5% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
1043869-98-2
(5-fluoro-2-methoxypyridin-4-yl)boronic acid

-
-
1453853-02-5
2-chloro-4-(5-fluoro-2-methoxypyridin-4-yl)pyrimidine

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium carbonate In 1,4-dioxane; water at 80℃; Suzuki Coupling; Inert atmosphere; | 99% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium carbonate In 1,4-dioxane; water at 80℃; Inert atmosphere; | 99% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
1262759-24-9
tert-butyl 3,5-dimethyl-2-oxoindoline-1-carboxylate

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In tetrahydrofuran at 50℃; for 18h; Schlenk technique; Inert atmosphere; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| In methanol at 0 - 20℃; | 98% |
| In methanol at 20℃; | 97% |
| In methanol at 0℃; for 2h; | 82% |
-
-
110-91-8
morpholine

-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
62968-37-0
4-(2-chloro-pyrimidin-4-yl)-morpholine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 55℃; for 2h; | 98% |
| With triethylamine In ethanol at 0℃; for 3h; | 90% |
| at 50℃; for 12h; | 88% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

-
-
221050-88-0
4-(2-chloropyrimidin-4-yl)piperazine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 19h; | 98% |
| With triethylamine In N,N-dimethyl-formamide at 20℃; | 95% |
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 4h; Product distribution / selectivity; | 90% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
29043-48-9
5-amino-2-methylbenzimidazole

-
-
191728-95-7
N-(2-chloropyrimidin-4-yl)-2-methyl-1H-benzo[d]imidazol-5-amine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; ethanol Reflux; Inert atmosphere; | 98% |
-
-
110-91-8
morpholine

-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
24192-96-9
4-(4-chloro-pyrimidine-2-yl)-morpholine

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 25℃; for 48h; | 98% |
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; | 53% |
| With triethylamine In ethanol at 0 - 20℃; |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
1292317-54-4
2-((tetrahydro-2H-pyran-4-yl)oxy)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile

-
-
1292317-55-5
5-(2-chloropyrimidin-4-yl)-2-(tetrahydro-2H-pyran-4-yloxy)benzonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate; tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane; water at 90℃; for 16h; | 98% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane; water at 90℃; for 16h; | 44% |
| With potassium carbonate; tetrakis(triphenylphosphine) palladium(0) In water; acetonitrile for 20h; Reflux; | 41% |
| With potassium carbonate; tetrakis(triphenylphosphine) palladium(0) In water; acetonitrile at 90℃; for 16h; Product distribution / selectivity; | 41% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
1307859-67-1
((3-(dibenzo[b,d]thiophen-4-yl)phenyl)boronic acid)

-
-
1383628-98-5
C22H13ClN2S

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene at 80℃; for 12h; | 98% |
-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium carbonate In 1,4-dioxane; water at 80℃; for 4h; Inert atmosphere; | 98% |
-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium carbonate In 1,4-dioxane; water at 100℃; for 3h; Inert atmosphere; | 98% |
-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In tetrahydrofuran at 50℃; for 18h; Schlenk technique; Inert atmosphere; regioselective reaction; | 98% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
75634-04-7
2-chloro-4-(4-methoxyphenyl)pyrimidine

| Conditions | Yield |
|---|---|
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In water; acetonitrile at 90℃; for 16h; | 97% |
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,4-dioxane; water; N,N-dimethyl-formamide at 80℃; for 10h; Inert atmosphere; | 87% |
| Stage #1: 2,6-Dichloropyrimidine; 4-methoxyphenylboronic acid With sodium carbonate In water for 0.25h; Suzuki-Miyaura Coupling; Inert atmosphere; Stage #2: With tetrakis(triphenylphosphine) palladium(0) In water Suzuki-Miyaura Coupling; Inert atmosphere; | 80% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol; water; toluene at 55℃; for 12h; Suzuki coupling; Inert atmosphere; regioselective reaction; |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
19335-11-6
1H-indazol-5-ylamine

-
-
1071105-22-0
N-(2-chloropyrimidin-4-yl)-1H-indazol-5-amine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Reflux; | 97% |
| With triethylamine In ethanol Temperature; Reflux; | 97% |
| With triethylamine In ethanol for 5h; Heating / reflux; | 80% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
6302-65-4
methyl 4-mercaptobenzoate

-
-
1042129-33-8
methyl 4-(2-chloropyrimidin-4-ylthio)benzoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 4-mercaptobenzoate With sodium hydroxide In methanol; water at 20℃; for 1h; Stage #2: 2,6-Dichloropyrimidine In methanol; water at 20℃; for 16.0833h; | 97% |
-
-
15344-56-6
2-(benzo[d]oxazol-2-yl)acetonitrile

-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: 2-(benzo[d]oxazol-2-yl)acetonitrile With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: 2,6-Dichloropyrimidine In tetrahydrofuran at 20℃; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 4℃; for 3h; | 97% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
97674-02-7
tributyl(1-ethoxyvinyl)stannane

-
-
932738-81-3
2-chloro-4-(1-ethoxyvinyl)pyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-Dichloropyrimidine; tributyl(1-ethoxyvinyl)stannane With bis-triphenylphosphine-palladium(II) chloride In N,N-dimethyl-formamide at 25 - 80℃; for 2h; Inert atmosphere; Stage #2: With potassium fluoride In ethyl acetate; N,N-dimethyl-formamide at 20℃; for 2h; | 97% |
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium fluoride In N,N-dimethyl-formamide at 85℃; for 4h; Inert atmosphere; | 80% |
| With bis-triphenylphosphine-palladium(II) chloride In N,N-dimethyl-formamide at 80℃; Stille coupling; | 79% |
| With bis-triphenylphosphine-palladium(II) chloride In N,N-dimethyl-formamide at 80℃; for 8h; | |
| With potassium fluoride; tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 20 - 100℃; for 2h; Inert atmosphere; |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
214360-60-8
N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetamide

-
-
945756-13-8
N-(4-(2-chloropyrimidin-4-yl)phenyl)acetamide

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In water; acetonitrile Reflux; Inert atmosphere; | 97% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In water; acetonitrile at 90℃; |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
60548-22-3
3-[(7-chloroquinolin-4-yl)amino]propan-1-ol

-
-
1526936-66-2
4-chloro-2-[(7-chloroquinolin-4-ylamino)propoxy]pyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 25℃; for 48h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: Cyclopropylmethanol With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 0.0833333h; Stage #2: 2,6-Dichloropyrimidine In tetrahydrofuran; mineral oil at 20℃; | 97% |
-
-
78242-20-3
1-methyl-1H-pyrazol-4-ol

-
-
3934-20-1
2,6-Dichloropyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 40℃; | 97% |
2,4-Dichloropyrimidine Chemical Properties
Structure of 2,4-Dichloropyrimidine (CAS NO.3934-20-1):
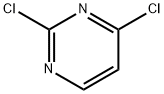
IUPAC Name: 2,4-dichloropyrimidine
Empirical Formula: C4H2Cl2N2
Molecular Weight: 148.9781
EINECS: 223-508-6
Index of Refraction: 1.559
Molar Refractivity: 32.22 cm3
Molar Volume: 99.7 cm3
Polarizability: 12.77×10-24cm3
Surface Tension: 51.5 dyne/cm
Density: 1.493 g/cm3
Flash Point: 104.3 °C
Enthalpy of Vaporization: 42.72 kJ/mol
Melting Point: 57-61 °C(lit.)
Boiling Point: 209.1 °C at 760 mmHg
Vapour Pressure: 0.298 mmHg at 25°C
Physical Appearance: White Solid
Product Categories: PYRIMIDINE;Pyrimidine Series;Pyridines, Pyrimidines, Purines and Pteredines;Halides;Pyrazines, Pyrimidines & Pyridazines;Heterocyclic Compounds;Building Blocks;Aromatics Compounds;Pyrimidines;Aromatics;Heterocycles;Pyrazines, Pyrimidines & Pyridazines;Building Blocks;Halogenated Heterocycles;Heterocyclic Building Blocks;PyrimidinesHeterocyclic Building Blocks
2,4-Dichloropyrimidine Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-28
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
R36:Irritating to eyes.
R28:Very toxic if swallowed.
RIDADR: 1759
WGK Germany: 3
HazardClass: 8
PackingGroup: III
2,4-Dichloropyrimidine Specification
2,4-Dichloropyrimidine , its cas register number is 3934-20-1. It also can be called 2,6-Dichloropyrimidine ; Pyrimidine, 2,4-dichloro- .
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 3934-23-4
- 39346-81-1
- 3934-77-8
- 3934-84-7
- 3934-87-0
- 393-49-7
- 393513-95-6
- 393516-77-3
- 393516-82-0
- 393-52-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View