-
Name
2-Amino-2-methyl-1-propanol
- EINECS 204-709-8
- CAS No. 124-68-5
- Article Data44
- CAS DataBase
- Density 0.93 g/cm3
- Solubility water: 0.1 M at 20 °C, clear, colorless
- Melting Point 24-28 °C(lit.)
- Formula C4H11NO
- Boiling Point 167.2 °C at 760 mmHg
- Molecular Weight 89.1374
- Flash Point 67.2 °C
- Transport Information 2735
- Appearance white crystals or viscous liquid
- Safety 61
- Risk Codes 36/38-52/53
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, F
F
- Synonyms AMP 75;AMP 90(amine);AMP Regular;Corrguard 75;Hydroxy-tert-butylamine;Isobutanol-2-amine;KV 5088;NSC 441;Pamabron;b-Aminoisobutanol;1,1-Dimethyl-2-hydroxyethylamine;2,2-Dimethylethanolamine;2-Amino-1-hydroxy-2-methylpropane;2-Amino-2,2-dimethylethanol;2-Aminoisobutanol;2-Hydroxy-1,1-dimethylethylamine;2-Hydroxymethyl-2-propylamine;2-Methyl-2-aminopropanol;AMP 100;2-Amino-2-methyl-1-propanol;
- PSA 46.25000
- LogP 0.41630
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 80℃; for 4h; | A 96% B 80% |

| Conditions | Yield |
|---|---|
| With tricresyl phosphate; hydrogen; palladium diacetate In diphenylether at 140℃; under 22502.3 - 60006 Torr; Autoclave; | 93% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 40 - 50℃; for 1h; pH=3 - 5.4; | 91% |
-
-
82922-13-2
2-(ethylamino)-2-methylpropanol hydrochloride

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
124-68-5
2-Amino-2-methyl-1-propanol

-
D
-
75-07-0
acetaldehyde

-
E
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With water at 25℃; Product distribution; Mechanism; anodic oxidation, carbonate buffer, pH 10; effect of substituents investigated with different types of β-alkanolamines; | A 50% B 44% C 14% D 26% E 66% |

-
-
82922-13-2
2-(ethylamino)-2-methylpropanol hydrochloride

-
A
-
50-00-0
formaldehyd

-
B
-
75-04-7
ethylamine

-
C
-
124-68-5
2-Amino-2-methyl-1-propanol

-
D
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With water at 25℃; anodic oxidation, pH 10, carbonate buffer; Further byproducts given; | A 50% B 44% C 14% D 66% |

-
-
76-39-1
2-nitro-2-methylpropanol

-
A
-
4706-13-2
N-(1-hydroxy-2-methylprop-2-yl)-hydroxylamine

-
B
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With citrate buffer; potassium chloride In ethanol at 30℃; cotrolled potential electrolysis, cathod: Hg, vs. S.C.E.; | A 64% B 25% |
-
-
86241-96-5
2-methyl-3-(vinyloxy)propan-2-amine

-
B
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With mercury(II) diacetate In benzene Heating; | A 3% B n/a |

| Conditions | Yield |
|---|---|
| With methanol; carbon dioxide; nickel at 70℃; under 25742.8 Torr; Hydrogenation; | |
| With methanol; nickel at 30℃; under 25742.8 Torr; Hydrogenation; | |
| With nickel Hydrogenation; |

| Conditions | Yield |
|---|---|
| With ethanol; nickel at 100℃; under 257428 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With tetrahydrofuran; lithium aluminium tetrahydride | |
| With lithium aluminium tetrahydride In tetrahydrofuran | |
| With sodium tetrahydroborate; iodine In tetrahydrofuran for 20h; Heating; | |
| With sodium tetrahydroborate; iodine In tetrahydrofuran Reflux; | |
| With lithium aluminium tetrahydride In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| (i) MeCN, Cl2, (ii) H2O; Multistep reaction; |
-
-
22451-34-9
2-ammonio-2-methyl-1-propanol cation

-
-
114444-46-1
C14H13NO

-
A
-
124225-44-1
1-methyl-4-(phenylacetyl)pyridinium cation

-
B
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; Rate constant; |

-
-
116221-74-0
N-chloro-2-amino-2-methylpropan-1-ol

-
A
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| In water at 25℃; Rate constant; Thermodynamic data; E(a); |

-
A
-
49642-07-1
statine

-
B
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 1h; Heating; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
87730-42-5
3-(N-succinimido)-2-propanol

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium tetrahydroborate In isopropyl alcohol |

| Conditions | Yield |
|---|---|
| at 100℃; under 250073 Torr; Hydrogenation; |

-
-
13257-67-5
2-amino-2-methylpropionic acid methyl ester

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With hydrogen; Nishimura catalyst <(45.9% Rh/19.9% Pt) oxide> In methanol at 25℃; under 75007.5 Torr; |
-
-
15028-41-8
methyl 2-aminoisobutyrate hydrochloride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; sodium hydrogencarbonate |
-
-
102520-97-8
2-((tert-butyloxycarbonyl)amino)-2-methylpropan-1-ol

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane |

-
B
-
124-68-5
2-Amino-2-methyl-1-propanol
-
-
497258-63-6
(+)-pinanediol (1R)-1-acetylamino-1-[3-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)phenyl]methylboronate

-
A
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 120℃; for 1h; |

| Conditions | Yield |
|---|---|
| at 125℃; Inert atmosphere; Schlenk technique; |
-
-
117052-89-8
N-(1-hydroxy-2-methylpropan-2-yl)-1-naphthamide

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 135℃; for 0.166667h; Microwave irradiation; |
-
-
32831-43-9
2-(hydroxymethyl)-2-methylpropylamine

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; sodium hydroxide In water at 0 - 70℃; for 0.333333h; Temperature; Hofmann Rearrangement; | 103 g |

| Conditions | Yield |
|---|---|
| With cerium(III) nitrate hexahydrate In methanol |
-
-
58004-55-0
N-(1-cyano-1-methyl-ethyl)formamide

-
A
-
682-85-9
N-(1-hydroxy-2-methylpropan-2-yl)formamide

-
B
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); hydrogen In 1,4-dioxane; water at 140℃; under 33753.4 Torr; for 18h; Reagent/catalyst; Pressure; Autoclave; | A n/a B 24 %Spectr. |
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); hydrogen In 1,4-dioxane; water at 20 - 150℃; under 41254.1 - 101260 Torr; for 48h; Autoclave; | A n/a B 73 %Chromat. |
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); water; hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In 1,4-dioxane at 150℃; under 41254.1 - 101260 Torr; for 48h; Catalytic behavior; Pressure; Reagent/catalyst; Temperature; Autoclave; | A 22 %Chromat. B 58.3 %Chromat. |

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 80℃; |
-
-
58004-55-0
N-(1-cyano-1-methyl-ethyl)formamide

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; water; hydrogen; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 140℃; under 41254.1 Torr; for 18h; Catalytic behavior; Reagent/catalyst; Pressure; Autoclave; | 24 %Spectr. |

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 50 - 120℃; for 3h; Product distribution / selectivity; | 100% |
| With chlorosulfonic acid In diethyl ether at 0℃; | 96% |
| With sulfuric acid In water at 150 - 170℃; for 4h; | 94% |
-
-
1121-60-4
pyridine-2-carbaldehyde

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
134777-99-4
4,4-dimethyl-2-pyridin-2-yl-1,3-oxazolidine

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Ambient temperature; | 100% |
| In ethanol at 20℃; for 3h; | 94% |
-
-
872-85-5
pyridine-4-carbaldehyde

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
134778-11-3
4-(4,4-Dimethyl-oxazolidin-2-yl)-pyridine

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Ambient temperature; | 100% |
-
-
500-22-1
3-pyridinecarboxaldehyde

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
134778-05-5
3-(4,4-Dimethyl-oxazolidin-2-yl)-pyridine

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Ambient temperature; | 100% |
-
-
93-89-0
benzoic acid ethyl ester

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
19312-05-1
N-(2-hydroxyl-1,1-dimethylethyl) benzamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-Amino-2-methyl-1-propanol With n-butyllithium; lanthanum(III) chloride In 1,2-dichloro-ethane at 0℃; Metallation; after the reagent addition stirring for 15 min at this temperature under nitrogen, than warming to reflux; Stage #2: benzoic acid ethyl ester for 1h; Amidation; reflux; | 100% |
| With sodium hydride 1.) toluene, RT, 1 h, 2.) toluene, 30 min; Yield given. Multistep reaction; | |
| With n-butyllithium; lanthanum(lll) triflate 1) toluene, 0 deg C, 15 min then 0 to 100 deg C, 2) toluene, reflux, 12 h; Yield given. Multistep reaction; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
102520-97-8
2-((tert-butyloxycarbonyl)amino)-2-methylpropan-1-ol

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Condensation; | 100% |
| With triethylamine In dichloromethane at 20℃; for 2.83333h; | 100% |
| Stage #1: di-tert-butyl dicarbonate With molybdenium(VI) dioxodichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-Amino-2-methyl-1-propanol In dichloromethane at 20℃; for 10h; | 99% |
-
-
96227-40-6
5-methoxy-3-methylbenzoic acid chloride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| In dichloromethane at 25℃; for 24h; | 100% |
-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
933-88-0
ortho-toluoyl chloride

-
-
95217-40-6
N-(1-hydroxy-2-methylpropan-2-yl)-2-methylbenzamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 100% |
| With triethylamine In dichloromethane at 0℃; | |
| With triethylamine In dichloromethane at 0℃; for 3h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
51805-99-3
2,4,4-trimethyl-2-pentyloxazolidine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |
-
-
502-56-7
5-Nonanone

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
174153-08-3
2,2-dibutyl-4,4-dimethyloxazolidine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |
-
-
927-49-1
dipentyl ketone

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
174153-09-4
4,4-dimethyl-2,2-dipentyloxazolidine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |
-
-
112-12-9
methyl nonyl ketone

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
69184-35-6
2,4,4-trimethyl-2-nonyloxazolidine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |
-
-
504-57-4
10-Nonadecanon

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
174153-10-7
4,4-dimethyl-2,2-dinonyloxazolidine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |
-
-
7169-12-2
2,3-dimethoxyterephthalic acid chloride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
179693-92-6
N,N'-bis(2-hydroxy-1,1-dimethylethyl)-2,3-dimethoxyterephthalamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2h; | 100% |
| In dichloromethane; water | 6.27 g (100%) |
-
-
69146-58-3
2,3-bis(benzyloxy)benzoyl chloride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
437613-96-2
2,3-dibenzyloxy-N-(2-hydroxy-1,1-dimethylethyl)benzamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 6.5h; | 100% |
-
-
14562-10-8
2-hydroxy-3-phenylbenzaldehyde

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| for 1h; Heating; | 100% |
-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
156660-22-9
2,2'-dihydroxybiphenyl-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| for 1h; Heating; | 100% |
-
-
108-24-7
acetic anhydride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
1569-96-6
N-(1-hydroxy-2-methylpropan-2-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride With molybdenium(VI) dioxodichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-Amino-2-methyl-1-propanol In dichloromethane at 20℃; for 0.5h; | 100% |
| Stage #1: acetic anhydride; 2-Amino-2-methyl-1-propanol In ethyl acetate at 20℃; for 18h; Inert atmosphere; Stage #2: With potassium carbonate In ethyl acetate at 20℃; Inert atmosphere; chemoselective reaction; | 98% |
| With Methylenediphosphonic acid at 20℃; for 1h; neat (no solvent); chemoselective reaction; | 95% |
| With sodium hydrogencarbonate In water at 20℃; for 16h; | |
| With triethylamine In chloroform at 20℃; for 24h; Temperature; chemoselective reaction; | 99 %Spectr. |
-
-
1538-75-6
2,2-dimethylpropanoic anhydride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
848598-54-9
N-(2-hydroxy-1,1-dimethylethyl)-2,2-dimethylpropionamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethylpropanoic anhydride With molybdenium(VI) dioxodichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-Amino-2-methyl-1-propanol In dichloromethane at 20℃; for 1h; | 100% |
-
-
98-88-4
benzoyl chloride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
19312-06-2
4,5-dihydro-4,4-dimethyl-2-phenoxazole

| Conditions | Yield |
|---|---|
| Stage #1: benzoyl chloride; 2-Amino-2-methyl-1-propanol In dichloromethane at 20℃; for 18h; Stage #2: With thionyl chloride In dichloromethane Further stages.; | 100% |
| Stage #1: benzoyl chloride; 2-Amino-2-methyl-1-propanol With triethylamine In dichloromethane at 0 - 20℃; for 10h; Stage #2: With thionyl chloride In chloroform at 0 - 20℃; for 24h; Stage #3: With sodium hydroxide In methanol at 0 - 20℃; for 12h; | 92% |
-
-
864296-43-5
3-methyl-4-isocyanato-benzoic acid methyl ester

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
864296-97-9
4-(3-[1,1-dimethyl-2-hydroxy-ethyl]-ureido)-3-methyl-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2h; | 100% |
| In tetrahydrofuran at 20℃; for 2h; |
-
-
860035-10-5
1-[[[(2,5-dioxopyrrolidin-1-yl)oxy]carbonyl]oxy]ethyl 2-methylpropanoate

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
1131570-15-4
[N-(2-hydroxy-tert-butyl)carbamoyloxy]ethyl 2-methylpropanoate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; acetonitrile at 20℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydride In mineral oil at 160℃; Inert atmosphere; | 100% |
| With sodium hydride at 140℃; for 4h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydride In mineral oil at 160℃; Inert atmosphere; | 100% |
-
-
78775-72-1
2-tert-butylmalonyl dichloride

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydride In mineral oil at 160℃; Inert atmosphere; | 100% |
2-Amino-2-methyl-1-propanol History
2-Amino-2-methyl-1-propanol Analytical Methods
2-Amino-2-methyl-1-propanol Specification
The CAS register number of 2-Amino-2-methyl-1-propanol is 124-68-5. It also can be called as 1-Propanol, 2-amino-2-methyl- and the IUPAC name about this chemical is 2-amino-2-methylpropan-1-ol. The molecular formula about this chemical is C4H11NO and the molecular weight is 89.14. It belongs to the following product categories, such as Industrial/Fine Chemicals; Chemical Amines; Aliphatics; Amines and so on. This chemical is stable and combustible. It incompatibles with strong oxidizing agents and it may present an explosion hazard if heated. This chemical can be used for the preparation of buffer solutions, suitable for the determination of alkaline phosphatase.
Physical properties about 2-Amino-2-methyl-1-propanol are:
(1)ACD/LogD (pH 5.5): -3.7; (2)ACD/LogD (pH 7.4): -3.1; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 2; (8)#H bond donors: 3; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 46.25Å2; (11)Index of Refraction: 1.447; (12)Molar Refractivity: 25.61 cm3; (13)Molar Volume: 95.7 cm3; (14)Polarizability: 10.15x10-24cm3; (15)Surface Tension: 34.4 dyne/cm; (16)Enthalpy of Vaporization: 47.01 kJ/mol; (17)Boiling Point: 167.2 °C at 760 mmHg; (18)Vapour Pressure: 0.566 mmHg at 25°C.
Preparation of 2-Amino-2-methyl-1-propanol:
2-Amino-2-methyl-1-propanol can be prepared by 2-methyl-2-nitro-propan-1-ol, this reaction can also produce b-hydroxyamino-isobutyl alcohol. This reaction will need reagent citrate buffer (pH 4.2), KCl and solvent ethanol. The reaction temperature of 30 ℃. The yield is about 25%. This reaction also need cotrolled potential electrolysis, cathod: Hg, vs. S.C.E.
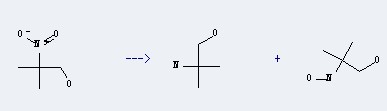
Uses of 2-Amino-2-methyl-1-propanol:
it can be used to produce 2-(2-hydroxy-ethylamino)-2-methyl-propan-1-o. This reaction will need reagent water.
Safety information of 2-Amino-2-methyl-1-propanol:
When you are using this chemical, please be cautious about it as the following:This chemical is irritating to eyes and skin and it is harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. When you are using it, you need avoid release to the environment. Refer to special instructions / safety data sheets.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(C)(CO)N
(2)InChI: InChI=1S/C4H11NO/c1-4(2,5)3-6/h6H,3,5H2,1-2H3
(3)InChIKey: CBTVGIZVANVGBH-UHFFFAOYSA-N
The toxicity data of 2-Amino-2-methyl-1-propanol is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 2150mg/kg (2150mg/kg) | Journal of the American College of Toxicology. Vol. 9(2), Pg. 203, 1990. | |
| rabbit | LDLo | oral | 1gm/kg (1000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Journal of Industrial Hygiene and Toxicology. Vol. 22, Pg. 315, 1940. |
| rat | LD50 | oral | 2900mg/kg (2900mg/kg) | Journal of the American College of Toxicology. Vol. 9(2), Pg. 203, 1990. |
Related Products
- 2-Amino-2-methyl-1-propanol
- 124691-76-5
- 124700-40-9
- 124700-41-0
- 124700-70-5
- 1247028-61-0
- 124-70-9
- 1247169-18-1
- 124727-10-2
- 124728-12-7
- 124728-90-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View