-
Name
2-Amino-4-chlorobenzoic acid
- EINECS 201-938-5
- CAS No. 89-77-0
- Article Data28
- CAS DataBase
- Density 1.476 g/cm3
- Solubility slightly soluble in water
- Melting Point 231-233 °C(lit.)
- Formula C7H6ClNO2
- Boiling Point 351.2 °C at 760 mmHg
- Molecular Weight 171.583
- Flash Point 166.2 °C
- Transport Information
- Appearance off-white powder
- Safety 26-36-37/39
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 4-Chloroanthranil acid;2-amino-4-chlorobenzoic-acid;2-amino-4-chloro-benzoate;Anthranilic acid, 4-chloro-;4-Chloroanthranilic acid;4-14-00-01072 (Beilstein Handbook Reference);4-Chloro-2-Aminobenzoic acid;2-amino-4-chloro-benzoic acid;Anthranilic acid, 4-chloro- (6CI,7CI,8CI);Benzoic acid, 2-amino-4-chloro-;
- PSA 63.32000
- LogP 2.20160
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 126 - 129℃; under 3800 - 9120 Torr; for 6h; | 88% |
| With ammonia; copper at 120℃; |
-
-
66909-52-2
2,4-dichlorobenzoic acid potassium salt

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper diacetate at 126 - 129℃; for 10h; | 68% |

| Conditions | Yield |
|---|---|
| With tin(II) chloride dihdyrate In ethanol for 1h; Inert atmosphere; | 23% |
| With platinum Hydrogenation; | |
| With ammonia; iron(II) sulfate |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide |

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
61680-22-6
7-chloro-3-(3-chlorophenyl)quinazoline-2,4(1H,3H)-dione

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
96385-49-8
4-chlorophthalamide

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| With alkaline aqueous potassium hypobromite |

| Conditions | Yield |
|---|---|
| With hydrogenchloride Zers. des entstandenen Hydrochlorids mit Natriumacetat; |
-
-
67-56-1
methanol

-
-
6341-92-0
6-chloroisatin

-
A
-
5900-58-3
2-amino-4-chloro-benzoic acid methyl ester

-
B
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium methylate 1.) 0-10 deg C 2.) room temperature, 30 min; Yield given. Multistep reaction; |


-
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; sodium hypochlorite |

| Conditions | Yield |
|---|---|
| at 120℃; |


| Conditions | Yield |
|---|---|
| at 120℃; |
-
-
96385-49-8
4-chlorophthalamide

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
A
-
91164-43-1
2-(2-Amino-5-chlor-benzoylamino)-5-chlor-benzoesaeure

-
B
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; sodium hypochlorite |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AlCl3; NaCl 2: aqueous KOH View Scheme |
-
-
34662-32-3
4-chloro-2-nitrobenzonitrile

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid 2: iron (II)-sulfate; ammonia View Scheme | |
| Multi-step reaction with 2 steps 1: iron; diluted acetic acid / <70 2: sulfuric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 2: sulfuric acid 3: iron (II)-sulfate; ammonia View Scheme | |
| Multi-step reaction with 3 steps 2: iron; diluted acetic acid / <70 3: sulfuric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium permanganate; magnesium sulfate 2: concentrated hydrochloric acid / Zers. des entstandenen Hydrochlorids mit Natriumacetat View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: nitric acid / water / 6 h / 60 °C / Cooling with ice 2: ammonium formate; 5%-palladium/activated carbon / ethanol / 6 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In ethanol for 1.5h; Reflux; |

| Conditions | Yield |
|---|---|
| at 200℃; for 1h; | 100% |
| at 200℃; for 2h; | 85% |
| With sodium hydroxide In water for 120h; Heating; | 33% |
-
-
127000-91-3
(2R,3R)-3-Amino-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
206350-07-4
2-Amino-4-chloro-N-[(1R,2R)-2-(2,4-difluoro-phenyl)-2-hydroxy-1-methyl-3-[1,2,4]triazol-1-yl-propyl]-benzamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide for 18h; Ambient temperature; | 100% |
| With 2,6-dimethylpyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 24h; Inert atmosphere; | 32 mg |
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 2h; |
-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
37585-16-3
(2-amino-4-chlorophenyl)methanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 100% |
| Stage #1: 2-Amino-4-chlorobenzoic acid With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 2h; Stage #2: With water; sodium hydroxide In tetrahydrofuran | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 2h; | 100% |
-
-
41979-39-9
4-piperidone hydrochloride

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
1451261-87-2
1-(2-amino-4-chlorobenzoyl)piperidin-4-one

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16h; | 100% |
-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
149-73-5
trimethyl orthoformate

-
-
31374-18-2
7-chloroquinazolin-4(1H)-one

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 120℃; for 2h; | 99% |
| With ammonium acetate In methanol at 120℃; for 3h; | 92% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; | 99% |
-
-
598-21-0
2-Bromoacetyl bromide

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
178270-85-4
2-(2-bromoacetamido)-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; N,N-dimethyl-formamide at 0 - 20℃; | 99% |
| In 1,4-dioxane; N,N-dimethyl-formamide at 0 - 20℃; | 99% |
| In 1,4-dioxane; N,N-dimethyl-formamide at 0 - 20℃; | 99% |
| In 1,4-dioxane; N,N-dimethyl-formamide at 20℃; |
-
-
108-94-1
cyclohexanone

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
5396-25-8
6,9-dichloro-1,2,3,4-tetrahydroacridine

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 3h; Heating / reflux; | 98.2% |
| With trichlorophosphate for 2h; Cyclization; condensation; Heating; | 93% |
| With trichlorophosphate for 2h; Heating; | 93% |
-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
20324-49-6
2-mercapto-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-Amino-4-chlorobenzoic acid With hydrogenchloride; sodium hydroxide; sodium nitrite In water at 0 - 5℃; for 0.5h; Stage #2: With potassium ethyl xanthogenate In water at 75 - 80℃; for 0.5h; Stage #3: With hydrogenchloride; sodium hydroxide; water; sodium hydrogensulfite more than 3 stages; | 98% |
| Stage #1: 2-Amino-4-chlorobenzoic acid With hydrogenchloride; sodium hydroxide; sodium nitrite In water at 0℃; for 1h; Stage #2: With potassium ethyl xanthogenate; sodium hydroxide In water at 65℃; | 63% |
| With hydrogenchloride; sodium disulfide; acetic acid; zinc; sodium nitrite Multistep reaction; |
-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
50419-88-0
2-amino-5-bromo-4-chlorobenzene carboxylic acid

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In acetonitrile at 20℃; for 1h; | 98% |
| With bromine In methanol at -15 - -10℃; for 2h; Concentration; | 92% |
| With bromine In methanol at -78℃; for 2h; | 62% |
-
-
108-24-7
acetic anhydride

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
708-73-6
7-chloro-2-methyl-4H-3,1-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| at 150℃; for 0.333333h; Microwave irradiation; Sealed tube; | 97% |
| for 2h; Acetylation; cyclization; condensation; Heating; | 81% |
| Reflux; | 81% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
5900-58-3
2-amino-4-chloro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 1h; | 97% |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
31374-18-2
7-chloro-3,4-dihydroquinazolin-4-one

| Conditions | Yield |
|---|---|
| With acetic acid; diethylamine at 150℃; for 2h; Product distribution / selectivity; | 96.1% |
| With montmorillonite K-10 for 0.0666667h; microwave irradiation; | 94% |
| at 130 - 160℃; for 5h; | 90.7% |
-
-
2131-55-7
4-Chlorophenyl isothiocyanate

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
118449-37-9
7-chloro-3-(4-chlorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 95% |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
31374-18-2
7-chloro-4-hydroxyquinazoline

| Conditions | Yield |
|---|---|
| at 160℃; for 4h; | 95% |
| 1.) 120 deg C, 2 h; 2.) 170 deg C, 2 h; | |
| at 200℃; for 6h; |
-
-
61464-95-7
(Chlorocarbonyl-phenyl-methyl)-carbamic acid benzyl ester

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
350238-17-4
2-(2-benzyloxycarbonylamino-2-phenyl-acetylamino)-4-chloro-benzoic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 95% |
-
-
89-77-0
2-Amino-4-chlorobenzoic acid

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 20℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate In N,N-dimethyl-formamide at 20℃; | 95% |
-
-
696-59-3
cis,trans-2,5-dimethoxytetrahydrofuran

-
-
7379-35-3
4-chlorpyridine hydrochloride

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
55540-33-5
1-(2-carboxy-4-chlorophenyl)pyrrole

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water | 95% |

-
-
64-19-7
acetic acid

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
929-06-6
2-(2-Aminoethoxy)ethanol

-
-
1268696-00-9
C13H15ClN2O3

| Conditions | Yield |
|---|---|
| With montmorillonite K 10 for 0.0333333h; Microwave irradiation; | 95% |

-
-
802294-64-0
propionic acid

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
929-06-6
2-(2-Aminoethoxy)ethanol

-
-
1268695-99-3
C14H17ClN2O3

| Conditions | Yield |
|---|---|
| With montmorillonite K 10 for 0.0333333h; Microwave irradiation; | 95% |

-
-
3473-63-0
formamidine acetic acid

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
31374-18-2
7-chloro-3,4-dihydroquinazolin-4-one

| Conditions | Yield |
|---|---|
| In water Microwave irradiation; | 95% |
| In 2-methoxy-ethanol at 120℃; for 16h; | 88.1% |
| In 2-methoxy-ethanol Reflux; | |
| In 2-methoxy-ethanol for 18h; Reflux; | |
| In 2-methoxy-ethanol at 130℃; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
40928-13-0
4-chloroisatoic anhydride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; Inert atmosphere; | 94.4% |
| In tetrahydrofuran at 20℃; for 18h; | 83% |
| With pyridine In dichloromethane; acetonitrile at 50℃; for 2h; | 74% |
-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
407-25-0
trifluoroacetic anhydride

-
-
42772-89-4
7-chloro-2-(trifluoromethyl)-4H-benzo[d][1,3]-oxazin-4-one

| Conditions | Yield |
|---|---|
| at 20℃; for 3h; | 94.4% |
-
-
67-56-1
methanol

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
5900-58-3
2-amino-4-chloro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid for 12h; Heating; | 94% |
| With thionyl chloride at -5℃; for 4.5h; Temperature; Reflux; Industrial scale; | 83% |
| With thionyl chloride at 0 - 5℃; for 24h; Reflux; | 82% |
-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
113290-36-1
methyl 4-chloro-2-(N,N-dimethyl-N'-formamidinyl)benzoate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Heating; | 94% |
-
-
3473-63-0
formamidine acetic acid

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
31374-18-2
7-chloroquinazolin-4(1H)-one

| Conditions | Yield |
|---|---|
| In ethyl methyl ether Reflux; | 94% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In toluene at 20℃; Cycloaddition; | 93% |
| In 1,4-dioxane at 40℃; for 1.5h; | |
| In 1,4-dioxane at 20℃; for 1h; | |
| In tetrahydrofuran; toluene at 20℃; for 24h; Inert atmosphere; |
-
-
98-88-4
benzoyl chloride

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
-
7033-52-5
7-chloro-2-phenyl-4H-benzo[d][1,3]oxazin-4-one

| Conditions | Yield |
|---|---|
| With pyridine for 0.5h; Acylation; cyclization; condensation; | 93% |
| With pyridine at 0 - 20℃; for 3h; | 70% |
| With pyridine at 20℃; for 12.5h; Time; Cooling with ice; | 60.2% |
-
-
75-44-5
phosgene

-
-
497-19-8
sodium carbonate

-
-
89-77-0
2-Amino-4-chlorobenzoic acid

-
A
-
40928-13-0
4-chloroisatoic anhydride

-
B
-
252232-80-7
2-amino-4-chloro-5-nitrobenzamide

| Conditions | Yield |
|---|---|
| In water; toluene | A 93% B n/a |

2-Amino-4-chlorobenzoic acid Chemical Properties
The Molecular formula of 2-Amino-4-chlorobenzoic acid(89-77-0) : C7H6ClNO2
The Molecular Weight of 2-Amino-4-chlorobenzoic acid(89-77-0): 171.58
Molecular Structure : 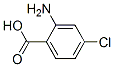
Melting point: 231-235 oC
WATER solubility: slightly soluble
Appearance: White or yellowish crystal
2-Amino-4-chlorobenzoic acid Uses
2-Amino-4-chlorobenzoic acid(89-77-0) is a intermediate for pharmaceuticals, and other organic products
2-Amino-4-chlorobenzoic acid Toxicity Data With Reference
Irritating to eyes, respiratory system and skin.The toxicological properties of 2-Amino-4-chlorobenzoic acid(89-77-0) have not been fully investigated.
Potential Health Effects
Eye: Causes eye irritation.
Skin: Causes skin irritation.
Ingestion: May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation: May cause irritation of the respiratory tract with burning pain in the nose and throat, coughing, wheezing, shortness of breath and pulmonary edema.
2-Amino-4-chlorobenzoic acid Safety Profile
The Hazard Codes of 2-Amino-4-chlorobenzoic acid(89-77-0) :  Xi
Xi
The Risk Statements information of 2-Amino-4-chlorobenzoic acid(89-77-0) :
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 2-Amino-4-chlorobenzoic acid(89-77-0):
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
37/39: Wear suitable gloves and eye/face protection
2-Amino-4-chlorobenzoic acid Standards and Recommendations
MOISTURE: 0.2% max
2-Amino-4-chlorobenzoic acid Specification
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View