-
Name
5-METHOXY-PYRIDIN-2-YLAMINE
- EINECS 125-856-9
- CAS No. 10167-97-2
- Article Data35
- CAS DataBase
- Density 1.139 g/cm3
- Solubility
- Melting Point 36-38 °C
- Formula C6H8N2O
- Boiling Point 251.6 °C at 760 mmHg
- Molecular Weight 124.142
- Flash Point 106 °C
- Transport Information
- Appearance dark red oil
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Pyridine,2-amino-5-methoxy- (7CI,8CI);5-Methoxy-2-aminopyridine;5-Methoxypyridin-2-amine;
- PSA 48.14000
- LogP 1.25360
Synthetic route
-
-
638352-78-0
1-(5-methoxypyridin-2-yl)-2,5-dimethyl-1H-pyrrole

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; triethylamine In ethanol; water for 20h; Heating / reflux; | 100% |
| With hydroxylamine hydrochloride; triethylamine In ethanol; water for 20h; Reflux; | 100% |
| With hydroxylamine hydrochloride; triethylamine In ethanol; water for 20h; Heating; | 81% |
-
-
76066-07-4
2-bromo-3-methoxy-6-nitropyridine

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With hydrazine; palladium 10% on activated carbon In ethanol for 0.75h; Heating / reflux; | 96% |
| With hydrazine; palladium 10% on activated carbon In ethanol; water for 0.75h; Heating / reflux; | 96% |
| With hydrazine hydrate; palladium on activated charcoal In ethanol for 1.5h; Heating; | 95% |
-
-
105170-27-2
2-bromo-5-methoxypyridine

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; lithium hexamethyldisilazane In 1,4-dioxane at 90℃; for 2h; Glovebox; Sealed tube; | 69% |
| With copper acetylacetonate; potassium phosphate; ammonia In N,N-dimethyl-formamide at 90℃; for 24h; Glovebox; Autoclave; chemoselective reaction; | 66% |

| Conditions | Yield |
|---|---|
| With sodium; copper at 160℃; for 48h; | 51% |
| With copper(l) iodide; 1,10-Phenanthroline; caesium carbonate at 110℃; Ullmann reaction; |

| Conditions | Yield |
|---|---|
| With copper In methanol at 100℃; for 12h; | 44% |
| With copper In methanol at 135℃; for 14h; | 36% |
| copper In methanol at 135℃; for 14h; Product distribution / selectivity; | 36% |
-
-
42900-89-0
3,4-dihydro-1H-2-benzopyran-3-ol

-
D
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 24h; Fischer Indole Synthesis; Inert atmosphere; | A 34% B 4% C 4% D 13% |


| Conditions | Yield |
|---|---|
| With copper In methanol at 160℃; | 31% |
-
-
477889-91-1
1-(5-iodopyridin-2-yl)-2,5-dimethyl-1H-pyrrole

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / copper(I) chloride / methanol; dimethylformamide / 2 h / 80 °C 2: 81 percent / hydroxylamine hydrochloride; triethylamine / ethanol; H2O / 20 h / Heating View Scheme |
-
-
24100-18-3
2-bromo-3-methoxypyridine

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80 percent / conc. H2SO4; fum. HNO3 / 4 h / 50 °C 2: 95 percent / N2H4*H2O / 5 percent Pd/C / ethanol / 1.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 48 percent / nitric acid, conc. sulfuric acid / 0.5 h / Heating 2: 78 percent / hydrazine hydrate / Pd/C / ethanol / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 71 percent / fuming HNO3, concd H2SO4 / 1 h / 55 °C 2: 59 percent / H2, NaOH / 10percent Pd/C / ethanol View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; nitric acid / 3 h / 55 °C 2: palladium on activated charcoal; hydrogen; potassium acetate / methanol; ethyl acetate / 48 h / 50 °C / 2585.81 Torr View Scheme |
-
-
20511-12-0
2-amino-5-iodopyridine

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol |

-
-
76066-07-4
2-bromo-3-methoxy-6-nitropyridine

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; palladium-carbon In ethanol; water | |
| With hydrazine hydrate; palladium-carbon In ethanol; water |
-
-
6602-32-0
2-bromo-pyridin-3-ol

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: diazomethyl-trimethyl-silane / dichloromethane; hexane / 3 h / 13 °C 2: sulfuric acid; nitric acid / 3 h / 55 °C 3: palladium on activated charcoal; hydrogen; potassium acetate / methanol; ethyl acetate / 48 h / 50 °C / 2585.81 Torr View Scheme |
-
-
228710-82-5
5-bromo-2-(2,5-dimethyl-pyrrol-1-yl)-pyridine

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: copper(l) iodide / N,N-dimethyl-formamide; methanol / 3 h / 80 °C 2: hydroxylamine hydrochloride; triethylamine / ethanol; water / 20 h / Reflux View Scheme |
-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
16182-04-0
Ethoxycarbonyl isothiocyanate

-
-
1092394-14-3
ethyl {[(5-methoxypyridin-2-yl)amino]carbonothioyl}carbamate

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
| at 20℃; | 100% |
-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
16182-04-0
Ethoxycarbonyl isothiocyanate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 20℃; | 100% |

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With pyridine; 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide In tetrahydrofuran for 2h; Reflux; | 99% |

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; thiourea In toluene at 70℃; Molecular sieve; Inert atmosphere; | 97% |
-
-
23386-72-3
2-bromo-1-(1-hydroxycyclohexyl)ethanone

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane at 110℃; for 12h; Sealed tube; Inert atmosphere; | 91% |
-
-
147410-78-4
3-(N-(4-chlorophenyl)sulfamoyl)benzoic acid

-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
1586822-35-6
3-(N-(4-chlorophenyl)sulfamoyl)-N-(5-methoxypyridin-2-yl)benzamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 89% |
-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
82-86-0
acenaphthene quinone

| Conditions | Yield |
|---|---|
| With oxygen; copper dichloride; Trimethylacetic acid In m-xylene; tert-butyl alcohol at 80 - 85℃; for 48h; | 86% |
-
-
62266-36-8
(+/-)-methylenecyclopropanecarboxylic acid

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 25℃; for 30h; | 85% |
-
-
127-19-5
N,N-dimethyl acetamide

-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
76066-09-6
N-(5-methoxy-2-pyridyl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl acetamide With 1,1'-carbonyldiimidazole at 120 - 125℃; for 0.5h; Inert atmosphere; Stage #2: 2-amino-5-methoxypyridine at 60 - 65℃; for 1.5h; Inert atmosphere; | 84% |
-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
97-51-8
5-Nitrosalicylaldehyde

| Conditions | Yield |
|---|---|
| In ethanol for 6h; Reflux; | 83% |
-
-
361473-14-5
3-(N-(4-chlorophenyl)-N-methylsulfamoyl)benzoic acid

-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
1586822-59-4
3-(N-(4-chlorophenyl)-N-methylsulfamoyl)-N-(5-methoxypyridin-2-yl)benzamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 82% |
-
-
90-02-8
salicylaldehyde

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| In ethanol for 6h; Reflux; | 82% |
-
-
14003-96-4
8-formyl-4-methyl-7-hydroxycoumarin

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| In methanol at 60℃; for 4h; | 81% |

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 50℃; for 2h; | 80% |
-
-
100-41-4
ethylbenzene

-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
869583-76-6
6-methoxy-2-phenylimidazolo[1,2-a]pyridine

| Conditions | Yield |
|---|---|
| Stage #1: ethylbenzene With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In water; ethyl acetate at 65℃; for 1.5h; Stage #2: 2-amino-5-methoxypyridine With sodium carbonate In water at 80℃; | 80% |
-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In acetone at 0 - 20℃; for 12.5h; | 80% |
-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
635-93-8
5-chlorosalicyclaldehyde

| Conditions | Yield |
|---|---|
| In ethanol for 6h; Reflux; | 79% |
-
-
32807-28-6
Methyl 4-chloroacetoacetate

-
-
10167-97-2
2-amino-5-methoxypyridine

-
-
1434288-35-3
2-(chloromethyl)-7-methoxy-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With polyphosphoric acid at 100℃; for 2h; Sealed tube; | 78% |
-
-
107-20-0
2-chloroethanal

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| In ethanol; water Reflux; | 77% |
-
-
672-13-9
2-hydroxy-5-methoxybenzaldehyde

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| In ethanol for 6h; Reflux; | 77% |

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane at 90℃; for 15h; Sealed tube; Inert atmosphere; | 76% |
-
-
504-02-9
1,3-cylohexanedione

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In water; toluene for 4h; Dean-Stark; Reflux; Inert atmosphere; | 76% |
-
-
30718-17-3
trimethylsilylmethyl isocyanide

-
-
123-08-0
4-hydroxy-benzaldehyde

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol at 130℃; for 0.833333h; Flow reactor; Green chemistry; | 76% |

-
-
1227281-44-8
3-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}-4-methoxy-4-oxobutanoic acid

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| Stage #1: 3-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}-4-methoxy-4-oxobutanoic acid With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide for 0.333333h; Stage #2: 2-amino-5-methoxypyridine In N,N-dimethyl-formamide at 20℃; for 16h; | 75% |
-
-
455-14-1
4-trifluoromethylphenylamine

-
-
1227281-44-8
3-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}-4-methoxy-4-oxobutanoic acid

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| Stage #1: 4-trifluoromethylphenylamine; 3-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}-4-methoxy-4-oxobutanoic acid With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide for 0.333333h; Stage #2: 2-amino-5-methoxypyridine In N,N-dimethyl-formamide at 20℃; for 16h; | 75% |


-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| Stage #1: (R)-4-((8-chloro-2,5-dioxo-3-(pyridin-2-ylmethyl)-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)methyl)-2-fluorobenzoic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In 1-methyl-pyrrolidin-2-one for 0.25h; Inert atmosphere; Microwave irradiation; Stage #2: 2-amino-5-methoxypyridine In 1-methyl-pyrrolidin-2-one at 80℃; for 0.0833333h; Inert atmosphere; Microwave irradiation; | 75% |

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With silver carbonate In 1,4-dioxane at 100℃; for 10h; Schlenk technique; Inert atmosphere; | 75% |
-
-
24115-90-0
2-nitro-3-benzyloxybenzoyl chloride

-
-
10167-97-2
2-amino-5-methoxypyridine

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 20h; | 74% |
2-Amino-5-methoxypyridine Chemical Properties
Following is the structure of 2-Amino-5-methoxypyridine (CAS NO.10167-97-2):
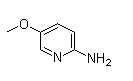
Molecular Formula: C6H8N2O
Molecular Weight: 124.14
Index of Refraction: 1.56
Molar Refractivity: 35.25 cm3
Molar Volume: 108.9 cm3
Density: 1.139 g/cm3
Flash Point: 106 °C
Surface Tension: 46.1 dyne/cm
Enthalpy of Vaporization: 48.9 kJ/mol
Boiling Point: 251.6 °C at 760 mmHg
Vapour Pressure: 0.0202 mmHg at 25 °C
Appearance of 2-Amino-5-methoxypyridine (CAS NO.10167-97-2): Dark Red Oil
Product Categories of 2-Amino-5-methoxypyridine (CAS NO.10167-97-2): Chemical Amines; Amines; Heterocycles
SMILES: O(c1cnc(N)cc1)C
InChI: InChI=1/C6H8N2O/c1-9-5-2-3-6(7)8-4-5/h2-4H,1H3,(H2,7,8)
InChIKey: XJKJHILCYUUVSJ-UHFFFAOYAQ
2-Amino-5-methoxypyridine Specification
2-Amino-5-methoxypyridine , its cas register number is 10167-97-2. It also can be called 5-Methoxy-pyridin-2-ylamine .
Related Products
- 2-Amino-5-methoxypyridine
- 10168-00-0
- 101682-68-2
- 1016854-64-0
- 1016859-98-5
- 101-68-8
- 10168-82-8
- 10169-02-5
- 10169-12-7
- 101691-65-0
- 101691-94-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View