-
Name
2-Benzoylpyridine
- EINECS 202-034-3
- CAS No. 91-02-1
- Article Data212
- CAS DataBase
- Density 1.139 g/cm3
- Solubility insoluble in water
- Melting Point 41-43 °C(lit.)
- Formula C12H9NO
- Boiling Point 317.7 °C at 760 mmHg
- Molecular Weight 183.21
- Flash Point 153.9 °C
- Transport Information
- Appearance colourless to slightly yellow crystalline chunks
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Ketone,phenyl 2-pyridyl (6CI,7CI,8CI);2-Pyridyl phenyl ketone;NSC20887;Phenyl 2-pyridyl ketone;Phenyl(2-pyridinyl)methanone;phenyl(pyridin-2-yl)methanone;methanone, phenyl-2-pyridinyl-;
- PSA 29.96000
- LogP 2.31260
Synthetic route
-
-
31796-72-2
phenyl(2-pyridinyl)methanol

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With butyltriphenylphosphonium chlorochromate In acetonitrile for 0.166667h; Heating; | 100% |
| With tert.-butylhydroperoxide; tetrabutyl-ammonium chloride; sodium carbonate; copper dichloride; 2,2′‐biquinoline‐4,4′‐dicarboxylic acid dipotassium salt In water at 40℃; for 48h; | 100% |
| With dicarbonyl(cyclopentadienyl)iron(II) chloride; sodium hydride In toluene for 20h; Catalytic behavior; Reagent/catalyst; Temperature; Reflux; Inert atmosphere; Schlenk technique; | 100% |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In chloroform at 0 - 80℃; for 1.16667h; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; tert.-butylhydroperoxide; iodine In water at 80℃; chemoselective reaction; | 99% |
| With oxygen In 1,2-dichloro-benzene at 110℃; under 760.051 Torr; for 6h; Schlenk technique; | 99% |
| With potassium bromate; cerium(IV) oxide In 1,4-dioxane; water; acetic acid at 95℃; for 4h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In toluene for 3h; | 99% |
| With oxygen; potassium carbonate In water; dimethyl sulfoxide Ambient temperature; | 48% |
| With oxygen; sodium hydride 1.) THF, 5 min; Yield given. Multistep reaction; |

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With benzyltriphenylphosphonium dichromate; silica gel for 0.166667h; | 98% |
| With 1-benzyl-4-aza-1-azoniabiyclo<2.2.2>octane peroxodisulfate In acetonitrile for 1h; Heating; | 96% |
| With benzyltriphenylphosphonium dichromate In acetonitrile for 0.5h; Oxidation; Heating; | 96% |
-
-
15260-65-8
2-(1-phenylvinyl)pyridine

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With oxygen at 110℃; for 8h; Sealed tube; | 97.3% |
| With oxygen at 110℃; for 8h; Schlenk technique; Green chemistry; | 95% |
| With 1-hydroxy-pyrrolidine-2,5-dione; graphitic carbon nitride; oxygen In acetonitrile at 20℃; Inert atmosphere; Irradiation; | 75% |

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With 3-carboxypyridinium chlorochromate In acetonitrile for 0.0333333h; Product distribution; Further Variations:; Reagents; Temperatures; without microwave irradiation; solvent-free; microwave irradiation; | 96% |
-
-
5029-67-4
2-iodopyridine

-
-
201230-82-2
carbon monoxide

-
-
98-80-6
phenylboronic acid

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With (bis(tricyclohexyl)phosphine)palladium(II) dichloride; potassium carbonate In tetrahydrofuran at 100℃; under 3750.3 Torr; for 20h; | 95% |
| Stage #1: 2-iodopyridine; phenylboronic acid With potassium carbonate In toluene for 0.166667h; Suzuki cross-coupling reaction; Autoclave; Stage #2: carbon monoxide In toluene at 100℃; under 10343.2 Torr; for 10h; Suzuki cross-coupling reaction; Autoclave; | 89% |
| With Bedford’s palladacycle; potassium carbonate; methoxybenzene at 120℃; under 3750.38 Torr; for 12h; Suzuki-Miyaura Coupling; Autoclave; | 89% |


| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; (18O)-dimethylsulfoxide at 20 - 120℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | A n/a B 95% |

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride; benzyltriphenylphosphonium peroxymonosulfate In acetonitrile for 3h; Heating; | 94% |
-
-
950993-49-4
2-bis(methylthio)phenylmethylpyridine

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With calcium carbonate; mercury dichloride In water; acetonitrile at 20℃; | 93% |
-
-
25726-04-9
phenylglyoxylyl chloride

-
-
13737-05-8
2-trimethylstannylpyridine

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; | 91% |
-
-
176173-93-6
(3,3-Dichloro-3,4,5,6-tetrahydro-pyridin-2-yl)-phenyl-methanone

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran for 3h; Heating; | 90% |
-
-
14178-31-5
(E)-2-pyridylphenyl ketoxime

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With iron(III) chloride In N,N-dimethyl-formamide at 25℃; for 0.5h; sonication; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: benzoic acid With isopropylmagnesium bromide In tetrahydrofuran; 2-methyltetrahydrofuran at 15℃; Inert atmosphere; Cooling with ice; Stage #2: 2-iodopyridine In tetrahydrofuran; 2-methyltetrahydrofuran Inert atmosphere; Stage #3: With TurboGrignard In tetrahydrofuran; 2-methyltetrahydrofuran for 0.166667h; Reagent/catalyst; Inert atmosphere; | 88% |
-
-
25726-04-9
phenylglyoxylyl chloride

-
-
17997-47-6
2-tri-n-butylstannylpyridine

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; | 87% |
-
-
201230-82-2
carbon monoxide

-
-
640-60-8
toluene-4-sulfonic acid phenyl ester

-
-
197958-29-5
pyridin-2-ylboronic acid

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; 1,2-bis-(diphenylphosphino)ethane In N,N-dimethyl acetamide at 80℃; under 760.051 Torr; for 6h; Suzuki-Miyaura Coupling; | 87% |


| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-pyridine With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Weinreb Ketone Synthesis; Stage #2: N-methoxy-N-methylcyanoformamide With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.33h; Weinreb Ketone Synthesis; Stage #3: phenylmagnesium bromide In tetrahydrofuran; hexane at -78℃; for 0.333333h; | 86% |


| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,4-dioxane for 12h; Reflux; | 86% |
-
-
101-82-6
2-Benzylpyridine

-
-
64-19-7
acetic acid

-
A
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
B
-
74031-79-1
phenyl(pyridin-2-yl)methyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: 2-Benzylpyridine; acetic acid With iodine for 0.0833333h; Stage #2: With tert.-butylhydroperoxide In decane at 20 - 100℃; for 1.5h; Reagent/catalyst; Solvent; Temperature; | A 10% B 85% |
| With copper(l) iodide; oxygen; palladium diacetate at 120℃; for 24h; Autoclave; |


| Conditions | Yield |
|---|---|
| With silver carbonate In acetonitrile at 60℃; for 1h; | 85% |
-
-
65007-00-3
pyridin-2-yl trifluoromethanesulfonate

-
-
201230-82-2
carbon monoxide

-
-
98-80-6
phenylboronic acid

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium(II) trifluoroacetate In tert-butyl methyl ether at 80℃; under 760.051 Torr; for 6h; Suzuki-Miyaura Coupling; | 85% |

-
-
201230-82-2
carbon monoxide

-
-
17763-67-6
Phenyl triflate

-
-
197958-29-5
pyridin-2-ylboronic acid

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium(II) trifluoroacetate In tert-butyl methyl ether at 80℃; under 760.051 Torr; for 6h; Suzuki-Miyaura Coupling; | 85% |


| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate In water at 120℃; for 18h; Green chemistry; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; water; silver nitrate at 20℃; Reagent/catalyst; | 85% |
| With tetrafluoroboric acid; oxygen; 9-(2-mesityl)-10-methylacridinium perchlorate In acetonitrile at 50℃; for 12h; Schlenk technique; Irradiation; | 60% |
-
-
5029-67-4
2-iodopyridine

-
-
67-66-3
chloroform

-
-
98-80-6
phenylboronic acid

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| With C33H27N2O3PPd; potassium hydroxide In toluene at 80℃; Suzuki-Miyaura Coupling; Sealed tube; | 85% |


| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; water at 20℃; Reagent/catalyst; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: benzoic acid With isopropylmagnesium bromide In tetrahydrofuran; 2-methyltetrahydrofuran at 15℃; Inert atmosphere; Cooling with ice; Stage #2: 2-bromo-pyridine In tetrahydrofuran; 2-methyltetrahydrofuran Inert atmosphere; Stage #3: With TurboGrignard In tetrahydrofuran; 2-methyltetrahydrofuran for 4h; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Stage #2: 2-Cyanopyridine In tetrahydrofuran at 0℃; | 82% |
| Stage #1: 2-Cyanopyridine; bromobenzene With magnesium In tetrahydrofuran at 0℃; Stage #2: With ammonium chloride In tetrahydrofuran | |
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Stage #2: 2-Cyanopyridine In tetrahydrofuran at 0℃; | |
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran at 20℃; Cooling; Stage #2: 2-Cyanopyridine In tetrahydrofuran at 0 - 20℃; Stage #3: With hydrogenchloride; water In diethyl ether for 0.5h; | |
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Stage #2: 2-Cyanopyridine In tetrahydrofuran at 0℃; Inert atmosphere; Stage #3: With water; ammonium chloride In tetrahydrofuran |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
31796-72-2
phenyl(2-pyridinyl)methanol

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate; polymer-bound NADH (2a) In acetonitrile; benzene at 80℃; for 120h; Further byproducts given; | 100% |
| With hydrogen; silver perchlorate; potassium hexamethylsilazane In toluene at 25℃; under 15001.5 Torr; for 17h; Reagent/catalyst; Glovebox; | 99% |
| With sodium tetrahydroborate In methanol at 10 - 20℃; for 1.5h; | 97% |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
74-89-5
methylamine

-
-
1182372-35-5
N-[phenyl(pyridin-2-yl)methylene]methanamine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 100% |
| In methanol for 7h; Reflux; |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
32838-55-4
2-benzylpiperidine

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; acetic acid at 20℃; under 760.051 Torr; for 8h; | 100% |
| Multi-step reaction with 2 steps 1: acetic acid; platinum on carbon; hydrogen / 6 h / 1551.49 - 2068.65 Torr 2: acetic acid; perchloric acid; platinum on carbon; hydrogen / 72 h / 50 - 55 Torr View Scheme |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
100-46-9
benzylamine

-
-
79159-65-2
1-phenyl-3-phenylimidazo[1,5-a]pyridine

| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In 1,2-dichloro-ethane for 5.5h; Solvent; Reagent/catalyst; Temperature; Reflux; | 99% |
| With copper(II) acetate monohydrate In N,N-dimethyl-formamide at 110℃; for 8h; Reagent/catalyst; Temperature; Solvent; | 93% |
| Stage #1: phenyl(pyridin-2-yl)methanone With manganese(IV) oxide; toluene-4-sulfonic acid at 20℃; for 0.25h; Inert atmosphere; Stage #2: benzylamine at 170℃; for 5.5h; Temperature; Inert atmosphere; Microwave irradiation; | 72% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.333333h; | 99% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C39H45FeN2O2PS; hydrogen; lithium tert-butoxide In isopropyl alcohol at 25 - 30℃; under 22502.3 Torr; for 12h; Reagent/catalyst; Autoclave; enantioselective reaction; | 99% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C37H41FeN2O2PS; hydrogen; lithium tert-butoxide In methanol at 40℃; under 22502.3 Torr; for 12h; Reagent/catalyst; Solvent; Temperature; Pressure; Autoclave; enantioselective reaction; | 97% |
| With C36H40Cl2N2P2Ru; potassium tert-butylate In dichloromethane; isopropyl alcohol at 23℃; for 2h; enantioselective reaction; | 92% |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
5583-33-5, 14159-57-0, 31796-72-2, 5583-34-6
(S)-(+)-α-phenyl-2-pyridylmethanol

| Conditions | Yield |
|---|---|
| With C60H60P2Rh(1+)*BF4(1-); hydrogen In dichloromethane at 20℃; under 6080.41 Torr; for 24h; Autoclave; enantioselective reaction; | 99% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C43H53FeN2O2PS; hydrogen; lithium tert-butoxide In isopropyl alcohol at 25 - 30℃; under 22502.3 Torr; for 12h; Catalytic behavior; Temperature; Reagent/catalyst; Solvent; Autoclave; enantioselective reaction; | 99% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C37H41FeN2O2PS; hydrogen; lithium tert-butoxide In methanol at 40℃; under 22502.3 Torr; for 12h; Reagent/catalyst; Pressure; Solvent; Temperature; Autoclave; enantioselective reaction; | 97% |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
888939-69-3
[tin(II)(CH(CMeN(2,6-iPr2C6H3)2)2(hydrido)]

-
-
1138335-32-6
[tin(II)(CH(CMeN(2,6-iPr2C6H3))2)(OCH(Ph)(2-Py))]

| Conditions | Yield |
|---|---|
| In toluene treatment of tin complex with 2-benzoylpyridine; | 99% |

| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In 1,2-dichloro-ethane for 2h; Solvent; Reflux; | 99% |
| With copper(II) acetate monohydrate In N,N-dimethyl-formamide at 110℃; for 8h; | 93% |

| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In 1,2-dichloro-ethane for 4h; Reflux; | 99% |
| With copper(II) acetate monohydrate In N,N-dimethyl-formamide at 110℃; for 8h; | 91% |
| Stage #1: phenyl(pyridin-2-yl)methanone With manganese(IV) oxide; toluene-4-sulfonic acid at 20℃; for 0.25h; Inert atmosphere; Stage #2: para-fluorobenzylamine at 170℃; for 5.5h; Inert atmosphere; Microwave irradiation; | 65% |

| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In 1,2-dichloro-ethane for 6h; Reflux; | 99% |
| With copper(II) acetate monohydrate In N,N-dimethyl-formamide at 110℃; for 8h; | 90% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
1346167-09-6
1-phenyl-3-(pyridin-2-yl)imidazo[1,5-a]pyridine

| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In 1,2-dichloro-ethane for 2h; Reflux; | 99% |
| Stage #1: phenyl(pyridin-2-yl)methanone With manganese(IV) oxide; toluene-4-sulfonic acid at 20℃; for 0.25h; Inert atmosphere; Stage #2: 2-(Aminomethyl)pyridine at 170℃; for 5.5h; Inert atmosphere; Microwave irradiation; | 41% |
| With copper(II) acetate monohydrate In N,N-dimethyl-formamide at 110℃; for 8h; | 31% |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
15260-65-8
2-(1-phenylvinyl)pyridine

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 20℃; for 1h; Stage #2: phenyl(pyridin-2-yl)methanone In tetrahydrofuran at 50℃; for 1h; | 99% |
| With potassium tert-butylate In tetrahydrofuran Inert atmosphere; | 50% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 20℃; for 1h; Wittig Olefination; Schlenk technique; Inert atmosphere; Stage #2: phenyl(pyridin-2-yl)methanone In tetrahydrofuran at 50℃; Wittig Olefination; Schlenk technique; Inert atmosphere; | 40% |
| With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 1h; | 21% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; Stage #2: phenyl(pyridin-2-yl)methanone In tetrahydrofuran; hexane at 20℃; for 12h; | 2.18 g |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
23702-98-9
alpha-phenyl-2-piperidinemethanol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethyl [2]alcohol at 60℃; under 15001.5 Torr; for 6h; | 98% |
| With platinum on carbon; hydrogen; acetic acid under 1551.49 - 2068.65 Torr; for 6h; | 80% |
| With hydrogenchloride; hydrogen; platinum(IV) oxide In ethanol under 41253.3 Torr; for 10h; | 100 % Spectr. |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

| Conditions | Yield |
|---|---|
| Stage #1: With pyridine; cinchonine In tetrahydrofuran at 0℃; for 0.5h; Reformatsky Reaction; Stage #2: phenyl(pyridin-2-yl)methanone In tetrahydrofuran at -40℃; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid at 118℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| In ethanol for 6h; Reflux; | 98% |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
917-54-4
methyllithium

-
-
19490-92-7
1-phenyl-1-(pyridin-2-yl)ethanol

| Conditions | Yield |
|---|---|
| In diethyl ether at -78 - 20℃; for 1h; Schlenk technique; Inert atmosphere; | 97% |
-
-
91-02-1
phenyl(pyridin-2-yl)methanone

-
-
917-54-4
methyllithium

-
A
-
37988-38-8
2-(pyridine-2-yl)propan-2-ol

-
B
-
19490-92-7
1-phenyl-1-(pyridin-2-yl)ethanol

| Conditions | Yield |
|---|---|
| In diethyl ether at -78 - 20℃; for 1.25h; | A 55% B 97% |


| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In 1,2-dichloro-ethane for 5h; Reflux; | 97% |
| With copper(II) acetate monohydrate In N,N-dimethyl-formamide at 110℃; for 8h; | 93% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.0833333h; | 97% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 60 - 62℃; for 0.25h; Temperature; Inert atmosphere; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| With ammonia; water; zinc In acetonitrile at 20℃; under 760.051 Torr; for 20h; Sealed tube; | 96% |
2-Benzoylpyridine Specification
The 2-Benzoylpyridine, with the CAS registry number 91-02-1 and EINECS registry number 202-034-3, has the systematic name of phenyl(pyridin-2-yl)methanone. It is a kind of colourless to slightly yellow crystalline chunks, and belongs to the product category of Pyridines derivates. And the molecular formula of this chemical is C12H9NO. What's more, it is often used in organic synthesis.
The physical properties of 2-Benzoylpyridine are as followings: (1)ACD/LogP: 1.88; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.88; (4)ACD/LogD (pH 7.4): 1.88; (5)ACD/BCF (pH 5.5): 15.74; (6)ACD/BCF (pH 7.4): 15.74; (7)ACD/KOC (pH 5.5): 250.31; (8)ACD/KOC (pH 7.4): 250.34; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 29.96 Å2; (13)Index of Refraction: 1.588; (14)Molar Refractivity: 54.13 cm3; (15)Molar Volume: 160.7 cm3; (16)Polarizability: 21.46×10-24cm3; (17)Surface Tension: 47 dyne/cm; (18)Density: 1.139 g/cm3; (19)Flash Point: 153.9 °C; (20)Enthalpy of Vaporization: 55.91 kJ/mol; (21)Boiling Point: 317.7 °C at 760 mmHg; (22)Vapour Pressure: 0.000379 mmHg at 25°C.
Preparation of 2-Benzoylpyridine: This chemical can be prepared by pyridine and N,N-dimethyl-benzamide. The reaction will need reagents BuLi and N,N-dimethylaminoethanol. And the yield is about 65%.
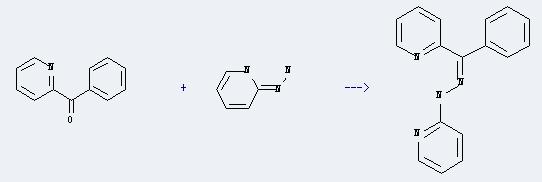
Uses of 2-Benzoylpyridine: It can react with 2-hydrazino-pyridine to produce N-(phenyl-pyridin-2-yl-methylene)-N'-pyridin-2-yl-hydrazine. This reaction will need reagent glacial acetic acid, and the solvent methanol. And the yield is about 70%.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin. Therefore, you had better take the following instructions: Wear suitable protective clothing, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(c1ccccc1)c2ncccc2
(2)InChI: InChI=1/C12H9NO/c14-12(10-6-2-1-3-7-10)11-8-4-5-9-13-11/h1-9H
(3)InChIKey: GCSHUYKULREZSJ-UHFFFAOYAK
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 475mg/kg (475mg/kg) | Journal of Medicinal Chemistry. Vol. 14, Pg. 551, 1971. |
Related Products
- 2-Benzoylpyridine
- 910232-84-7
- 910247-97-1
- 910309-12-5
- 91031-31-1
- 910-31-6
- 91031-88-8
- 91037-65-9
- 910378-55-1
- 91037-91-1
- 910400-60-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View