-
Name
2-Fluoro-6-nitrophenol
- EINECS 216-199-4
- CAS No. 1526-17-6
- Article Data11
- CAS DataBase
- Density 1.511 g/cm3
- Solubility
- Melting Point 92-94 ºC
- Formula C6H4FNO3
- Boiling Point 201.5 ºC at 760 mmHg
- Molecular Weight 157.101
- Flash Point 75.6 ºC
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 6-Fluoro-2-nitrophenol;NSC 10282;
- PSA 66.05000
- LogP 1.96270
Synthetic route

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid; bismuth subnitrate/charcoal In dichloromethane at 20℃; for 1h; regioselective reaction; | 85% |
| With nitric acid In dichloromethane at 0℃; for 1h; | |
| With nitric acid In dichloromethane at 0 - 5℃; for 1h; | |
| With nitric acid In dichloromethane at 0℃; for 1h; |
-
-
402-67-5
3-fluoro-1-nitrobenzene

-
A
-
1526-17-6
2-fluoro-6-nitrophenol

-
B
-
403-19-0
2-fluoro-4-nitrophenol

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; potassium tert-butylate; ammonia In tetrahydrofuran for 0.25h; | A 7% B 76% |

| Conditions | Yield |
|---|---|
| With Nitrogen dioxide In pentane 35 min, 0 deg C then 20 min, room temperature; | A 47% B 49% |
-
-
367-12-4
2-fluorophenol

-
A
-
1526-17-6
2-fluoro-6-nitrophenol

-
B
-
681227-38-3
fluoro-[1,4]benzoquinone-4-oxime

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite at 0℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite at 0℃; |
-
-
367-12-4
2-fluorophenol

-
A
-
1526-17-6
2-fluoro-6-nitrophenol

-
B
-
403-19-0
2-fluoro-4-nitrophenol

-
C
-
123871-61-4
6-fluoro-6-nitrocyclohexa-2,4-dienone

| Conditions | Yield |
|---|---|
| With nitric acid In acetic anhydride at -60℃; Title compound not separated from byproducts; | |
| With nitric acid In acetic anhydride Title compound not separated from byproducts; |


-
-
1526-17-6
2-fluoro-6-nitrophenol

| Conditions | Yield |
|---|---|
| With steam at 170 - 190℃; |
-
-
367-12-4
2-fluorophenol

-
-
7440-44-0
pyrographite

-
A
-
1526-17-6
2-fluoro-6-nitrophenol

-
B
-
403-19-0
2-fluoro-4-nitrophenol

| Conditions | Yield |
|---|---|
| With nitric acid In hexane; dichloromethane | A 13.5 g (30 %) B n/a |
| With nitric acid In hexane; dichloromethane | A 13.5 g (30 %) B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: 2-fluoro-6-nitrophenol With potassium hydroxide In ethanol for 1h; Stage #2: ethyl bromoacetate In N,N-dimethyl-formamide for 24h; Further stages.; | 99% |
| With potassium carbonate In acetonitrile at 90℃; for 2h; | 74.7% |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
1526-17-6
2-fluoro-6-nitrophenol

-
-
122455-35-0
2-fluoro-6-nitrophenyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With potassium carbonate | 98% |
| With potassium carbonate In acetone at 20℃; for 4h; | 40% |
| Stage #1: 2-fluoro-6-nitrophenol With potassium carbonate In acetone at 20℃; for 0.333333h; Stage #2: trifluoromethylsulfonic anhydride In acetone at 20℃; for 4h; | 40% |
| With pyridine In dichloromethane at 0℃; for 2h; |
-
-
1526-17-6
2-fluoro-6-nitrophenol

-
-
96-32-2
bromoacetic acid methyl ester

-
-
698984-51-9
(2-fluoro-6-nitrophenoxy)acetic acid, methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 65℃; for 3h; | 98% |
| With potassium carbonate In acetone for 5h; Reflux; | 93% |
-
-
1526-17-6
2-fluoro-6-nitrophenol

-
-
598-72-1
2-Bromopropionic acid

-
-
698984-51-9
(2-fluoro-6-nitrophenoxy)acetic acid, methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 5h; Heating / reflux; | 93% |
-
-
1526-17-6
2-fluoro-6-nitrophenol

-
-
53981-25-2
2-amino-6-fluoro phenol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol for 1h; | 87% |
| With tin(ll) chloride In tetrahydrofuran; water Reflux; | 55.3% |
| Stage #1: 2-fluoro-6-nitrophenol With water; tin(ll) chloride In tetrahydrofuran at 80℃; for 2h; Stage #2: With sodium hydrogencarbonate In water | 12% |
| With ammonium formate; palladium 10% on activated carbon In methanol at 20℃; for 0.5h; | |
| With hydrogen; nickel In methanol under 760.051 Torr; |

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In dichloromethane at 0 - 20℃; for 1.5h; | 83% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; calcium carbonate In N,N-dimethyl-formamide at 100℃; for 6h; | 83% |
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 6h; | 83% |
-
-
453-20-3
3-Hydroxytetrahydrofuran

-
-
1526-17-6
2-fluoro-6-nitrophenol

-
-
917909-41-2
3-(2-fluoro-6-nitrophenoxy)-tetrahydrofuran

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In dichloromethane at 20℃; for 20h; | 68% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | A 58% B n/a |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | A 54% B n/a |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | A 50% B n/a |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 49% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 46% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | A n/a B 46% |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 43% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | A 43% B n/a |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | A 42% B n/a |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 41% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 39% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 39% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 39% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 38% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | A 37% B n/a |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 35% |
-
-
1526-17-6
2-fluoro-6-nitrophenol

-
-
59524-02-6
N-tert-butoxycarbonyl-L-serine benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-fluoro-6-nitrophenol With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at -3℃; for 0.166667h; Stage #2: N-tert-butoxycarbonyl-L-serine benzyl ester at 20℃; for 16h; | 30% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In ethyl acetate at 25℃; for 0.12h; Schlenk technique; | 18% |
2-Fluoro-6-nitrophenol Specification
The 2-Fluoro-6-nitrophenol, with the CAS registry number 1526-17-6, is also known as Phenol, 2-fluoro-6-nitro-. It belongs to the product category of Fluorobenzene. Its EINECS registry number is 216-199-4. This chemical's molecular formula is C6H4FNO3 and molecular weight is 157.10. What's more, its IUPAC name is the same with its product name. It should be kept in a cold and dry place.
Physical properties about 2-Fluoro-6-nitrophenol are: (1)ACD/LogP: 1.84; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.61; (4)ACD/LogD (pH 7.4): 0.12; (5)ACD/BCF (pH 5.5): 8.77; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 142.25; (8)ACD/KOC (pH 7.4): 4.53; (9)#H bond acceptors: 4; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 55.05 Å2; (13)Index of Refraction: 1.581; (14)Molar Refractivity: 34.67 cm3; (15)Molar Volume: 103.9 cm3; (16)Surface Tension: 56.4 dyne/cm; (17)Density: 1.511 g/cm3; (18)Flash Point: 75.6 °C; (19)Enthalpy of Vaporization: 45.56 kJ/mol; (20)Boiling Point: 201.5 °C at 760 mmHg; (21)Vapour Pressure: 0.217 mmHg at 25 °C.
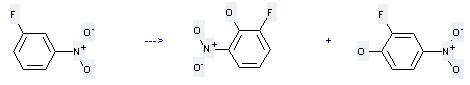
Preparation of 2-Fluoro-6-nitrophenol: this chemical can be prepared by 1-Fluoro-3-nitro-benzene. This reaction needs reagents t-BuOK, t-BuOOH, NH3 and solvent tetrahydrofuran. The reaction time is 15 min. The yield is 76 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. If swallowed, it's harmful to health. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And in case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: Fc1cccc([N+]([O-])=O)c1O
(2) InChI: InChI=1S/C6H4FNO3/c7-4-2-1-3-5(6(4)9)8(10)11/h1-3,9H
(3) InChIKey: HIGRXCJEFUYRNW-UHFFFAOYSA-N
Related Products
- 2-Fluoro-6-nitrophenol
- 152617-91-9
- 152623-93-3
- 152-62-5
- 152626-78-3
- 152628-00-7
- 152628-01-8
- 152628-02-9
- 152628-03-0
- 15262-86-9
- 15263-52-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View