-
Name
2-Hydroxy-4-methoxybenzaldehyde
- EINECS 211-604-0
- CAS No. 673-22-3
- Article Data100
- CAS DataBase
- Density 1.231 g/cm3
- Solubility Solubility in methanol is almost transparent. Insoluble in water.
- Melting Point 41-43 °C(lit.)
- Formula C8H8O3
- Boiling Point 271.5 °C at 760 mmHg
- Molecular Weight 152.15
- Flash Point 112.1 °C
- Transport Information
- Appearance creamy white to beige or light brown cryst. powder
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms p-Anisaldehyde,2-hydroxy- (6CI,7CI,8CI);2-Formyl-5-methoxyphenol;2-Hydroxy-p-anisaldehyde;4-Methoxy-6-hydroxybenzaldehyde;4-Methoxysalicylaldehyde;NSC 155334;o-Hydroxy-p-methoxybenzaldehyde;
- PSA 46.53000
- LogP 1.21330
Synthetic route

| Conditions | Yield |
|---|---|
| With triethylamine; magnesium chloride In tetrahydrofuran Inert atmosphere; Reflux; | 98% |
| With triethylamine; magnesium chloride In tetrahydrofuran Inert atmosphere; Reflux; regioselective reaction; | 92% |
| Stage #1: O-methylresorcine With triethylamine; magnesium chloride In acetonitrile at 20℃; for 0.25h; Stage #2: formaldehyd In acetonitrile Reflux; | 89% |
-
-
59648-29-2
2-(hydroxymethyl)-5-methoxyphenol

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With oxygen In ethanol at 20℃; under 760.051 Torr; for 0.316667h; Catalytic behavior; Green chemistry; | 98% |
| With dihydrogen peroxide at 20℃; for 3.5h; Catalytic behavior; Green chemistry; | 98% |
| With air; (acryl-TEMPO)-co-(allylamine-treated chlorophyll b-Co(III) complex) immobilized on SiO2-covered Fe3O4 magnetic nanoparticles In water at 25℃; for 0.333333h; | 94% |
| With oxygen In aq. buffer at 45℃; for 13h; pH=4.5; Reagent/catalyst; Green chemistry; | 93 %Chromat. |
-
-
954374-77-7
ethyl (E)-3-(2-formyl-5-methoxyphenoxy)acrylate

-
-
2298-07-9
4-Bromo-1-naphthylamine

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; | A n/a B 95% |

-
-
954374-77-7
ethyl (E)-3-(2-formyl-5-methoxyphenoxy)acrylate

-
-
106-49-0
p-toluidine

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
954374-93-7
C30H33NO8

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; | A n/a B 95% |


| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at -5 - 25℃; | 94% |
| With boron trichloride Ambient temperature; | 93% |
| With boron trichloride In dichloromethane at 0 - 20℃; for 16h; Dealkylation; | 93% |
-
-
50-00-0
formaldehyd

-
-
150-19-6
O-methylresorcine

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
700-44-7
6-methoxysalicylaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine; magnesium chloride In acetonitrile for 2h; Heating; | A 91% B 5% |
| With triethylamine; magnesium chloride |


| Conditions | Yield |
|---|---|
| Stage #1: 2-iodo-5-methoxyphenol With iodine; triethylamine; triphenylphosphine In dichloromethane; toluene at 20℃; for 0.0833333h; Sealed tube; Green chemistry; Stage #2: formic acid In dichloromethane; toluene at 70℃; for 1.5h; Sealed tube; Green chemistry; | 91% |
-
-
20503-09-7
2-(2-hydroxy-4-methoxyphenyl)acetic acid

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With benzyltriphenylphosphonium peroxodisulfate In acetonitrile for 0.5h; Oxidation; decarboxylation; Heating; | 90% |
-
-
954374-77-7
ethyl (E)-3-(2-formyl-5-methoxyphenoxy)acrylate

-
-
108-42-9
3-chloro-aniline

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; | A n/a B 90% |

-
-
954374-77-7
ethyl (E)-3-(2-formyl-5-methoxyphenoxy)acrylate

-
-
75-31-0
isopropylamine

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; | A n/a B 89% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 86.3% |
| With potassium carbonate In acetone for 5h; Reflux; | 55% |
| With potassium carbonate In acetone for 5h; Reflux; | 55% |
-
-
40138-18-9
(2-formyl-5-methoxyphenyl)boronic acid

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-p-toluidine N-oxide In dichloromethane at 20℃; for 0.0166667h; | 86% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In toluene at 90℃; for 10h; | 83% |

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; bis-[(trifluoroacetoxy)iodo]benzene In 1,2-dichloro-ethane at 100℃; for 8h; Catalytic behavior; Reagent/catalyst; Temperature; Schlenk technique; Inert atmosphere; chemoselective reaction; | 72% |
| With boric acid tributyl ester; dihydrogen peroxide; sec.-butyllithium 1.) THP, 20 deg C, 45 min, 2.) 0 deg C, 1 h; Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1.1: sodium sulfate / methanol / 24 h / Reflux 2.1: tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; oxygen / acetone / 0.5 h / 20 °C / Glovebox 2.2: 0.5 h / 70 °C / Glovebox View Scheme |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 2h; Heating; | 69% |
| With potassium carbonate In acetone at 20℃; Inert atmosphere; | 68.4% |
| With potassium carbonate In acetone at 20℃; for 24h; | 64% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 1h; Heating; | 65% |
-
-
30114-41-1
3-methoxyphenyl formate

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With boron tribromide In 1,2-dichloro-ethane at -10 - 20℃; for 23h; Fries rearrangement; | 59% |
-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
-
74-88-4
methyl iodide

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
613-45-6
2,4-Dimethoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In acetone at 23℃; for 18h; | A 53% B n/a |
| With potassium carbonate; β‐cyclodextrin In acetone for 3h; Product distribution; Heating; var. β-cyclodextrin derivatives and reagent ratio; | A 63.6 % Spectr. B 36.4 % Spectr. |

-
-
67-71-0
dimethylsulfone

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
613-45-6
2,4-Dimethoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetone for 10h; Reflux; | A 51% B 33% |

-
-
123-11-5
4-methoxy-benzaldehyde

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; bis-[(trifluoroacetoxy)iodo]benzene In 1,2-dichloro-ethane at 80℃; for 8h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | A 43% B 9% |
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; potassium acetate; bis-[(trifluoroacetoxy)iodo]benzene In 1,2-dichloro-ethane at 80℃; for 8h; Reagent/catalyst; Temperature; Schlenk technique; Inert atmosphere; | A 5% B 32% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; | 40% |
| With diethyl ether |
-
-
13198-99-7
3-hydroxy-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
77184-86-2
1-(2-hydroxy-4-methoxyphenyl)-2-(4-methoxyphenyl)ethanedione

-
C
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With manganese triacetate In acetic acid Heating; Further byproducts given; | A 30% B 8% C 34 % Spectr. D 4% |
| With manganese triacetate In acetic acid Heating; Further byproducts given; | A 3% B 8% C 34 % Spectr. D 4% |

-
-
150-19-6
O-methylresorcine

-
-
4885-02-3
Dichloromethyl methyl ether

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
700-44-7
6-methoxysalicylaldehyde

-
C
-
18278-34-7
4-hydroxy-2-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: O-methylresorcine With titanium tetrachloride In dichloromethane at 0℃; for 1h; Inert atmosphere; Stage #2: Dichloromethyl methyl ether In dichloromethane at 0℃; for 0.75h; Inert atmosphere; Overall yield = 61 %; | A n/a B n/a C 17% |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
60-29-7
diethyl ether

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
613-45-6
2,4-Dimethoxybenzaldehyde

-
-
150-19-6
O-methylresorcine

-
-
74-90-8
hydrogen cyanide

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
18278-34-7
4-hydroxy-2-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; diethyl ether und Erhitzen des Reaktionsprodukts mit Wasser; | |
| With hydrogenchloride | |
| With hydrogenchloride; zinc(II) chloride | |
| With hydrogenchloride; aluminium trichloride | |
| With hydrogenchloride Darst.; |

-
-
150-19-6
O-methylresorcine

-
-
67-66-3
chloroform

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
18278-34-7
4-hydroxy-2-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide Nebenprod. 2: zwei isomeren Resorcindialdehydmonomethylaethern; |


-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride |
-
-
150-19-6
O-methylresorcine

-
-
864131-95-3
N,N'-diphenylformamidine

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| at 185℃; und Kochen des Reaktionsprodukts mit Natronlauge; |
-
-
77-78-1
dimethyl sulfate

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
A
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
B
-
18278-34-7
4-hydroxy-2-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With methanol; sodium carbonate |


| Conditions | Yield |
|---|---|
| With water; sodium carbonate at 70 - 80℃; | |
| With sodium ethanolate In ethanol | |
| With potassium hydrogencarbonate In N,N-dimethyl-formamide for 3h; Etherification; Heating; | |
| With potassium carbonate; potassium iodide In acetone for 48h; Reflux; | |
| With potassium hydrogencarbonate; potassium iodide In acetone for 48h; Reflux; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide In tetrahydrofuran; water at 20℃; for 1h; Dakin reaction; | 100% |
| With 7,8-difluoro-1,3-dimethyl-5-ethyl-4a-hydroperoxyalloxazine; dihydrogen peroxide; sodium hydrogencarbonate In methanol; water at 20℃; for 0.166667h; Dakin oxidation; | 95% |
| With dihydrogen peroxide at 20℃; for 0.833333h; Dakin Phenol Oxidation; Green chemistry; | 94% |
| With dihydrogen peroxide In water at 20℃; for 2.5h; Dakin Phenol Oxidation; Green chemistry; | 92% |
| With sodium percarbonate In tetrahydrofuran; water for 2h; ultrasonication; | 83% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
139386-27-9
ethyl 3-(2-hydroxy-4-methoxyphenyl)-(E)-2-propenoate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.5h; Wittig Olefination; | 100% |
| In dichloromethane at 20℃; | 100% |
| In toluene at 20℃; Inert atmosphere; | 90% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
106-96-7
propargyl bromide

-
-
236390-57-1
4-methoxy-2-[(prop-2-yn-1-yl)oxy]benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 3h; Inert atmosphere; | 99% |
| Stage #1: 2-Hydroxy-4-methoxybenzaldehyde With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.25h; Inert atmosphere; Stage #2: propargyl bromide In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 95% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
93198-68-6
(E)-methyl 2’-hydroxy-4’-methoxycinnamate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 95℃; for 5h; Wittig reaction; | 100% |
| In dichloromethane at 0 - 20℃; for 15h; Wittig Olefination; Inert atmosphere; | 93% |
| In toluene at 150℃; for 0.166667h; Wittig Olefination; Inert atmosphere; Microwave irradiation; Sealed tube; stereoselective reaction; | 89% |
| In toluene at 20℃; Inert atmosphere; | 86% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
57497-39-9
N-tertbutylhydroxylamine hydrochloride

-
-
255052-05-2
α-(2-hydroxy-4-methoxyphenyl)-N-tert-butylnitrone

| Conditions | Yield |
|---|---|
| With pyrrolidine In methanol; water at 20℃; for 16h; | 100% |
| With magnesium oxide at 20℃; for 0.0916667h; Neat (no solvent); Grinding; | 86% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
20439-47-8
(1R,2R)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In methanol | 100% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
13139-86-1
4-methoxyphenyl magnesium bromide

-
-
433331-87-4
2-hydroxy-4,4'-dimethoxydiphenylmethanol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In dichloromethane at 0 - 20℃; for 18h; Mitsunobu Displacement; | 100% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
192711-11-8
2-((tert-butyldimethylsilyl)oxy)-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With 1H-imidazole; tert-butyldimethylsilyl chloride In dichloromethane at 20℃; Inert atmosphere; | 100% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
137362-04-0
3-Methoxy-6-(1'-hydroxypentyl)phenol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; Alkylation; | 99.5% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
17861-16-4
2-hydroxy-4-methoxybenzaldehyde oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium hydroxide In ethanol; water at 0 - 20℃; | 99% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water for 3h; Reflux; | 83% |
| With hydroxylamine hydrochloride; potassium carbonate In water Reflux; | 77% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
108-24-7
acetic anhydride

-
-
62536-84-9
2-formyl-5-methoxyphenyl acetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 1h; | 99% |
| In pyridine for 17h; | 70% |
| With pyridine at 20℃; for 24h; | |
| With pyridine at 20℃; for 4h; | |
| With pyridine |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
197015-32-0
4-methoxy-2-trifluoromethanesulfonyloxybenzaldehyde

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; | 95% |
| With triethylamine | 90% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
107-15-3
ethylenediamine

-
-
157982-82-6
N,N′-bis(4-methoxysalicylidene)-1,2-diaminoethane

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Inert atmosphere; Reflux; | 99% |
| In methanol Reflux; | 84% |
| In ethanol at 20℃; for 6h; | 80% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
490038-99-8
1-(bromoacetyl)-3-propylazulene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 9.5h; Heating; | 99% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
2065-66-9
methyl-triphenylphosphonium iodide

-
-
522592-59-2
2-hydroxy-4-methoxy-styrene

| Conditions | Yield |
|---|---|
| Stage #1: methyl-triphenylphosphonium iodide With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 2h; Stage #2: 2-Hydroxy-4-methoxybenzaldehyde In tetrahydrofuran; hexane at -78℃; Wittig reaction; | 99% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
16649-50-6
N-tert-Butylhydroxylamine

-
-
255052-05-2
α-(2-hydroxy-4-methoxyphenyl)-N-tert-butylnitrone

| Conditions | Yield |
|---|---|
| for 0.0583333h; Ionic liquid; Microwave irradiation; | 99% |
| 72.9% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
7518-21-0
2-(1-methyl-1H-indol-3-yl)ethylamine

-
-
407-25-0
trifluoroacetic anhydride

| Conditions | Yield |
|---|---|
| Stage #1: 2-Hydroxy-4-methoxybenzaldehyde; 2-(1-methyl-1H-indol-3-yl)ethylamine In chloroform at -40℃; for 8h; Molecular sieve; Stage #2: trifluoroacetic anhydride In chloroform at -40℃; Molecular sieve; | 99% |

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; | 99% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
1121-22-8, 1436-59-5, 20439-47-8, 21436-03-3, 41013-43-8, 694-83-7
trans-1,2-Diaminocyclohexane

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.166667h; | 99% |
| In ethanol at 20℃; for 6h; | 80% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
52814-92-3
1-(6-methoxybenzofuran-2-yl)ethan-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; chloroacetone In acetone for 24h; Inert atmosphere; Reflux; | 99% |
| Multi-step reaction with 2 steps 1: caesium carbonate / N,N-dimethyl-formamide / 18 h / 22 °C 2: toluene-4-sulfonic acid / tetrahydrofuran / 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With Fe3O4(at)SiO2(at)Propyl-7-aminonaphthalene-1,3-disulfonic acid In water for 0.583333h; Reflux; | 99% |


| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 24h; Inert atmosphere; | 99% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In acetonitrile for 8h; Reflux; | 98.7% |
| With piperidine In ethanol |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
57543-36-9
5-bromo-2-hydroxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With bromine In dichloromethane at -20℃; for 0.333333h; Inert atmosphere; regioselective reaction; | 98% |
| With 1,3-di-n-butyl-1H-imidazol-3-ium tribromide at 20℃; Neat (no solvent); regioselective reaction; | 98% |
| With bromine; acetic acid at 0 - 20℃; for 2h; | 90% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
105-36-2
ethyl bromoacetate

-
-
76322-06-0
2-ethoxycarbonylmethoxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2-Hydroxy-4-methoxybenzaldehyde With sodium carbonate In acetone for 0.5h; Large scale; Stage #2: ethyl bromoacetate In acetone for 2h; Reagent/catalyst; Reflux; Large scale; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 97% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
106-95-6
allyl bromide

-
-
71186-58-8
2-allyloxy-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide; benzyltri(n-butyl)ammonium chloride In dichloromethane Ambient temperature; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide | 98% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
155912-26-8
1-[4-((E)-4-methoxyl-2-hydroxybenzylidene)]oxime

| Conditions | Yield |
|---|---|
| With pyridine; hydroxylamine hydrochloride In ethanol at 60℃; for 3h; Inert atmosphere; | 98% |
| With N-hydroxyphthalimide In water at 90℃; for 3h; Sealed tube; | 90% |
| With hydroxylamine hydrochloride; sodium hydroxide In ethanol; water at 0 - 20℃; for 1h; | 71% |
| With pyridine; hydroxylamine hydrochloride In ethanol Condensation; | |
| With hydroxylamine hydrochloride; triethylamine In ethanol; ethyl acetate |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
190451-39-9
(1R,2S,4aR,8aR)-2-hydroxy-decahydro-5,5,8a-trimethyl-1-naphthylmethanol

| Conditions | Yield |
|---|---|
| With sulfuric acid In dimethyl sulfoxide at 20℃; for 1.5h; | 98% |
2-Hydroxy-4-methoxybenzaldehyde Chemical Properties
Product Name: Benzaldehyde,2-hydroxy-4-methoxy- (CAS NO.673-22-3)
IUPAC Name: 2-hydroxy-4-methoxybenzaldehyde
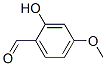
Molecular Formula: C8H8O3
Formula Weight: 152.15
MP: 41-43 °C(lit.)
Density: 1.231 g/cm3
Boiling Point: 271.5 °C at 760 mmHg
Flash Point: 112.1 °C
Vapour Pressure: 0.00387 mmHg at 25°C
Index of Refraction: 1.587
Sensitive : Air Sensitive
Physical State: Solid
Color: slightly beige
Product Categories: FINE Chemical & INTERMEDIATES; Aromatic Aldehydes & Derivatives (substituted); Benzaldehyde
2-Hydroxy-4-methoxybenzaldehyde Uses
Benzaldehyde,2-hydroxy-4-methoxy- (CAS NO.673-22-3) is used as pharmaceutical intermediate.
2-Hydroxy-4-methoxybenzaldehyde Toxicity Data With Reference
| 1. | sce-hmn:lyms 500 µmol/L | MUREAV Mutation Research. 206 (1988),17. |
2-Hydroxy-4-methoxybenzaldehyde Consensus Reports
Reported in EPA TSCA Inventory.
2-Hydroxy-4-methoxybenzaldehyde Safety Profile
Safety: CAUTION: May irritate eyes, skin, and respiratory tract
Hazard Codes : Xi (Irritant)
Risk Statements : 36/37/38 (Irritating to eyes, respiratory system and skin)
Safety Statements : 26-36-24/25 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice; Wear suitable protective clothing; Avoid contact with skin and eyes)
WGK Germany : 3
RTECS : BZ2810000
F : 10 (Keep under argon)
Hazard Note : Irritant
HS Code : 29124900
Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating vapors.
Related Products
- 2-Hydroxy-4-methoxybenzaldehyde
- 6732-85-0
- 67330-25-0
- 67330-62-5
- 673-31-4
- 673-32-5
- 67333-70-4
- 67336-19-0
- 67337-03-5
- 67338-35-6
- 67338-65-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View