-
Name
3-(2,6-Dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride
- EINECS 224-723-8
- CAS No. 4462-55-9
- Article Data11
- CAS DataBase
- Density 1.472 g/cm3
- Solubility 22.98mg/L at 25℃
- Melting Point 92 °C
- Formula C11H6Cl3NO2
- Boiling Point 390.9 °C at 760 mmHg
- Molecular Weight 290.533
- Flash Point 190.2 °C
- Transport Information
- Appearance Yellow to brown powder
- Safety 26-36/37/39-45-36/37/39/45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolylcarbonylchloride;
- PSA 43.10000
- LogP 4.33580
Synthetic route
-
-
3919-76-4
3-(2,6-dichloro-phenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; N,N-dimethyl-formamide at 60℃; | 94% |
| With phosphorus pentachloride at 45℃; for 1h; | 84% |
| With thionyl chloride |
-
-
83-38-5
2,6-dichlorobenzaldehyde

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) NH2OH, (ii) Cl2, CHCl3, (iii) /BRN= 506727/, MeOH 2: aq. KOH / ethanol 3: SOCl2 View Scheme | |
| Multi-step reaction with 5 steps 1.1: hydroxylamine sulfate / methanol / 0.5 h 1.2: 0.5 h / pH 8 - 9 / Reflux 2.1: chlorine / methanol / 0 - 5 °C 3.1: sodium hydroxide / methanol / 0.5 h / 0 - 5 °C / pH 10 3.2: 2 h / 0 - 20 °C / pH 9 4.1: water; sodium hydroxide / methanol / 1 h / pH 11 - 13 / Reflux 5.1: phosphorus pentachloride / 1 h / 45 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: hydroxylamine sulfate / methanol / 1 h / 20 - 40 °C 1.2: 24 h / 0 - 20 °C / pH ~ 9.5 / Inert atmosphere 1.3: pH ~ 7 - 7.5 2.1: phosphorus pentachloride / toluene View Scheme | |
| Multi-step reaction with 5 steps 1: hydroxylamine hydrochloride; sodium hydroxide / water; ethanol / 0 - 90 °C 2: N-chloro-succinimide / N,N-dimethyl-formamide / 2 h / 20 °C 3: triethylamine / dichloromethane / 0 - 20 °C 4: lithium hydroxide monohydrate; water / ethanol / 50 °C 5: thionyl chloride; N,N-dimethyl-formamide / 60 °C View Scheme |

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. KOH / ethanol 2: SOCl2 View Scheme | |
| Multi-step reaction with 2 steps 1: water; sodium hydroxide / methanol / 1 h / pH 11 - 13 / Reflux 2: phosphorus pentachloride / 1 h / 45 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / water; methanol / 3 h 2: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 40 °C View Scheme |
-
-
6579-27-7
2,6-dichlorobenzohydroximoyl chloride

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydroxide / methanol / 0.5 h / 0 - 5 °C / pH 10 1.2: 2 h / 0 - 20 °C / pH 9 2.1: water; sodium hydroxide / methanol / 1 h / pH 11 - 13 / Reflux 3.1: phosphorus pentachloride / 1 h / 45 °C View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 0 - 20 °C 2: lithium hydroxide monohydrate; water / ethanol / 50 °C 3: thionyl chloride; N,N-dimethyl-formamide / 60 °C View Scheme |
-
-
25185-95-9
2,6-dichlorobenzaldoxime

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: chlorine / methanol / 0 - 5 °C 2.1: sodium hydroxide / methanol / 0.5 h / 0 - 5 °C / pH 10 2.2: 2 h / 0 - 20 °C / pH 9 3.1: water; sodium hydroxide / methanol / 1 h / pH 11 - 13 / Reflux 4.1: phosphorus pentachloride / 1 h / 45 °C View Scheme | |
| Multi-step reaction with 4 steps 1: N-chloro-succinimide / N,N-dimethyl-formamide / 2 h / 20 °C 2: triethylamine / dichloromethane / 0 - 20 °C 3: lithium hydroxide monohydrate; water / ethanol / 50 °C 4: thionyl chloride; N,N-dimethyl-formamide / 60 °C View Scheme |
-
-
24248-21-3
ethyl 3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazole-4-carboxylate

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium hydroxide monohydrate; water / ethanol / 50 °C 2: thionyl chloride; N,N-dimethyl-formamide / 60 °C View Scheme |
-
-
551-16-6
6-Aminopenicillanic Acid

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
343-55-5
sodium dicloxacillin

| Conditions | Yield |
|---|---|
| Stage #1: 6-Aminopenicillanic Acid With sodium hydroxide In water at 0 - 5℃; for 0.333333h; Stage #2: 3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride In acetone at 5℃; for 1h; pH=7; Stage #3: With sodium 2-ethylhexanoic acid In isopropyl alcohol at 30℃; for 0.5h; Temperature; Solvent; | 80.21% |
| With ammonium hydroxide In ethyl acetate pH=2 - 8; |
-
-
107-19-7
propargyl alcohol

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
661452-05-7
prop-2-ynyl 3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 79% |
-
-
937681-62-4
3,4-dihydro-6-t-butyl-2H-1,4-benzoxazine

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 40℃; for 0.416667h; Microwave irradiation; | 70% |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
71472-58-7
3,4-dihydro-7-methyl-2H-1,4-benzoxazine

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 40℃; for 0.416667h; Microwave irradiation; | 60% |
-
-
105655-01-4
6-bromo-3, 4-dihydro-2H-benzo[β][1, 4]oxazine

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 40℃; for 0.416667h; Microwave irradiation; | 55% |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
70558-11-1
6-chloro-3,4-dihydro-2H-benzo[b][1,4]oxazine

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 40℃; for 0.416667h; Microwave irradiation; | 54% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene at 40℃; for 0.416667h; Microwave irradiation; | 53% |
-
-
21224-16-8
2-amino-6-ethylbenzo[d]thiazole

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 60℃; for 18.5h; Inert atmosphere; | 51% |
-
-
7493-63-2
anthranilic acid allyl ester

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
848187-10-0
2-{[3-(2,6-Dichloro-phenyl)-5-methyl-isoxazole-4-carbonyl]-amino}-benzoic acid allyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran at 20℃; | 49% |
| With sodium hydrogencarbonate In tetrahydrofuran; water at 20℃; |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
143557-91-9
(1R,3S,5S)-tert-butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 30℃; | 41% |
-
-
17557-67-4
2-amino-6-(methylsulfonyl)benzothiazole

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; N,N-dimethyl-formamide at 40 - 45℃; for 24h; Inert atmosphere; | 37% |

| Conditions | Yield |
|---|---|
| With crithmene; potassium ethyl xanthogenate; sodium phosphate In dichloromethane at 60℃; for 24h; Giese Free Radical Synthesis; Irradiation; | 35% |
-
-
1416231-32-7
9b-amino-4b-hydroxy-7-isopropyl-4b,9b-dihydro-10H-indeno[1,2-b]benzofuran-10-one

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
1416233-43-6
3-(2,6-dichlorophenyl)-N-(4b-hydroxy-7-isopropyl-10-oxo-9b,10-dihydro-4bH-benzo[d]indeno[1,2-b]furan-9b-yl)-5-methylisooxazole-4-carboxamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 19% |
-
-
6494-19-5
3-methyl-6-nitro-1H-indazole

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
62235-36-3
1-[3-(2,6-dichloro-phenyl)-5-methyl-isoxazole-4-carbonyl]-3-methyl-6-nitro-1H-indazole

| Conditions | Yield |
|---|---|
| With triethylamine In benzene |
-
-
62-53-3
aniline

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
4402-85-1
3-(2,6-dichloro-phenyl)-5-methyl-isoxazole-4-carboxylic acid anilide

| Conditions | Yield |
|---|---|
| In acetone |
-
-
6325-22-0
chlorhydrate de (β-aminoethyl)-4 benzenesulfonamide

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
24477-34-7
3-(2,6-dichloro-phenyl)-5-methyl-isoxazole-4-carboxylic acid 4-sulfamoyl-phenethylamide

| Conditions | Yield |
|---|---|
| In pyridine |
-
-
22179-80-2
2,4-dichlorobenzamidoxime

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 25℃; for 24h; Acylation; |
-
-
180468-20-6
(2R,3R)-3-amino-1-(1'-methoxycarbonyl-2'-methylpropenyl)-4-oxoazetidine-2-sulfonic acid tetrabutylammonium salt

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2.16667h; Condensation; |
-
-
180468-23-9
(2R,3S)-3-amino-1-(1'-methoxycarbonyl-2'-methylpropenyl)-4-oxoazetidine2-sulfonic acid

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2.16667h; Condensation; |
-
-
124-40-3
dimethyl amine

-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
349131-41-5
3-(2,6-Dichlorophenyl)-N,N,5-trimethylisoxazole-4-carboxamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
848187-11-1
2-{[3-(2,6-Dichloro-phenyl)-5-methyl-isoxazole-4-carbonyl]-amino }-benzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 49 percent / NaHCO3 / tetrahydrofuran / 20 °C 2: 100 percent / Pd(PPh3)4; morpholine / CH2Cl2 / 20 °C View Scheme |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 49 percent / NaHCO3 / tetrahydrofuran / 20 °C 2: 100 percent / Pd(PPh3)4; morpholine / CH2Cl2 / 20 °C 3: TBTU; DIPEA / dimethylformamide / 20 °C View Scheme |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 49 percent / NaHCO3 / tetrahydrofuran / 20 °C 2: 100 percent / Pd(PPh3)4; morpholine / CH2Cl2 / 20 °C 3: TBTU; DIPEA / dimethylformamide / 20 °C View Scheme |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 49 percent / NaHCO3 / tetrahydrofuran / 20 °C 2: 100 percent / Pd(PPh3)4; morpholine / CH2Cl2 / 20 °C 3: TBTU; DIPEA / dimethylformamide / 20 °C View Scheme |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 49 percent / NaHCO3 / tetrahydrofuran / 20 °C 2: 100 percent / Pd(PPh3)4; morpholine / CH2Cl2 / 20 °C 3: TBTU; DIPEA / dimethylformamide / 20 °C View Scheme |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 49 percent / NaHCO3 / tetrahydrofuran / 20 °C 2: 100 percent / Pd(PPh3)4; morpholine / CH2Cl2 / 20 °C 3: TBTU; DIPEA / dimethylformamide / 20 °C View Scheme |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
52320-56-6
(4-{2-[3-(2,6-dichloro-phenyl)-5-methyl-isoxazole-4-carbonylamino]-ethyl}-benzenesulfonyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: 1,2-dimethoxy-ethane View Scheme |
-
-
4462-55-9
3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolylcarbonyl chloride

-
-
52320-40-8
3-(2,6-dichloro-phenyl)-5-methyl-isoxazole-4-carboxylic acid 4-(azepan-1-ylcarbamoyl-sulfamoyl)-phenethylamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine 2: 1,2-dimethoxy-ethane 3: 1,2-dimethoxy-ethane View Scheme |
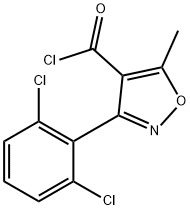
3-(2,6-Dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride Chemical Properties
IUPAC Name: 3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazole-4-carbonyl chloride
Empirical Formula: C11H6Cl3NO2
Molecular Weight: 290.5298g/mol
EINECS: 224-723-8
Structure of 4-Isoxazolecarbonylchloride, 3-(2,6-dichlorophenyl)-5-methyl- (CAS NO.4462-55-9):
Index of Refraction: 1.584
Molar Refractivity: 66.09 cm3
Molar Volume: 197.3 cm3
Polarizability: 26.2×10-24cm3
Surface Tension: 48.9 dyne/cm
Density: 1.472 g/cm3
Flash Point: 190.2 °C
Enthalpy of Vaporization: 64.04 kJ/mol
Melting Point: 92 °C
Boiling Point: 390.9 °C at 760 mmHg
Vapour Pressure: 2.57E-06 mmHg at 25°C
Product Categories: Oxazole&Isoxazole
Canonical SMILES: CC1=C(C(=NO1)C2=C(C=CC=C2Cl)Cl)C(=O)Cl
InChI: InChI=1S/C11H6Cl3NO2/c1-5-8(11(14)16)10(15-17-5)9-6(12)3-2-4-7(9)13/h2-4H,1H3
InChIKey: IZQGELJKDARDMZ-UHFFFAOYSA-N
3-(2,6-Dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride Safety Profile
Hazard Codes:  C
C
Risk Statements: 34
R34:Causes burns.
Safety Statements: 26-36/37/39-45-36/37/39/45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: 3261
Hazard Note: Corrosive
3-(2,6-Dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride Specification
4-Isoxazolecarbonylchloride, 3-(2,6-dichlorophenyl)-5-methyl- , its cas register number is 4462-55-9. It also can be called 3-(2,6-Dichlorophenyl)-5-methylisoxazole-4-carboxylic acid chloride . 4-Isoxazolecarbonylchloride, 3-(2,6-dichlorophenyl)-5-methyl- (CAS NO.4462-55-9) is a yellow to brown powde which may cause burns.
Related Products
- 3-(2-(1,3-DIOXO-2-METHYLINDANYL))-GLUTARIMIDE
- 3-(2-(1,3-DIOXO-2-PHENYL-4,5,6,7-TETRA-HYDRO-4,7-DITHIAINDANYL))GLUTARIMIDE
- 3-(2-(1,3-DIOXO-2-PHENYLINDANYL))-GLUTARIMIDE
- 3-(2-(2,4-Di-tert-pentylphenoxy)acetamido)-N-(5-oxo-1-(2,4,6-trichlorophenyl)-2-pyrazolin-3-yl)benzamide
- 3-(2-(4-CHLOROBENZYLAMINO)ETHYL)INDOLE MONOHYDROCHLORIDE
- 3-(2-(5-BROMO-2-PYRIDYLOXY)ETHYL) THIAZOLIDINE HYDROCHLORIDE
- 3-(2-(DIETHYLAMINO)ETHYL)-5-(2-FURYL)-1-PHENYL-1H-PYRAZOLINE HYDROCHLORIDE
- 3-(2-(DIETHYLPYRROLIDINO)ETHOXY)-6-METHOXY-2-PHENYLBENZOFURAN HYDROCHLORIDE
- 3-(2-(Dimethylamino)ethyl)indole-sulfosalicylate
- 3-(2-(Trifluoromethyl)benzyloxy)-5-(5,6-dimethoxy-1H-benzo[d]imidazol-1-yl)thiophene-2-carboxamide
- 446263-96-3
- 446-26-4
- 446273-59-2
- 446275-89-4
- 446284-32-8
- 446286-90-4
- 446286-92-6
- 446286-94-8
- 446287-05-4
- 446292-04-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View