-
Name
3,3,3-Trifluoropropionic acid
- EINECS 629-437-0
- CAS No. 2516-99-6
- Article Data27
- CAS DataBase
- Density 1.437 g/cm3
- Solubility
- Melting Point 9.7 °C(lit.)
- Formula C3H3F3O2
- Boiling Point 128 °C at 760 mmHg
- Molecular Weight 128.051
- Flash Point 31.2 °C
- Transport Information UN 3265 8/PG 2
- Appearance Clear colorless liquid
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Propionicacid, 3,3,3-trifluoro- (7CI,8CI);3,3,3-Trifluoropropanoic acid;b,b,b-Trifluoropropionic acid;
- PSA 37.30000
- LogP 1.02340
Synthetic route

| Conditions | Yield |
|---|---|
| With tetrabutoxytitanium; sodium molybdate; dihydrogen peroxide; calcium chloride In ethanol; water at 75 - 90℃; for 6h; Time; Inert atmosphere; | 96.1% |
| With nitric acid; sodium nitrite In water at 0 - 20℃; for 4h; | 61% |
| With sulfuric acid; sodium nitrite In water at 0 - 20℃; for 3h; | 61% |
-
-
85345-75-1
3,3,3-trifluoropropionyl fluoride

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With water at 5 - 20℃; for 0.5h; | 93.4% |
| With water; sodium hydroxide at 5 - 20℃; for 0.5h; | 90.2% |
-
-
18830-44-9
methyl 3,3,3-trifluoropropionate

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 5 - 30℃; for 0.5h; | 88% |
| With hydrogen bromide | 80% |
-
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

-
-
431-54-9
3,3,3-trifluoro-2-chloropropionyl chloride

-
A
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
B
-
110230-36-9
2-chloro-3,3,3-trifluoropropanoic acid

| Conditions | Yield |
|---|---|
| With water at 50℃; for 4h; Product distribution / selectivity; | A 84.5% B n/a |

-
-
460-40-2
3,3,3-trifluoropropanal

-
A
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
B
-
936107-87-8
3,3,3-trifluoro-1-(3,3,3-trifluoro-1-hydroxypropoxy)propan-1-ol

-
C
-
936107-88-9
C9H9F9O3

| Conditions | Yield |
|---|---|
| With water at -10 - 25℃; for 0.166667 - 168h; Product distribution / selectivity; | A 0.06% B 70.9% C 0.2% |

-
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With water at 50℃; for 5h; Product distribution / selectivity; | 70.5% |
-
-
460-40-2
3,3,3-trifluoropropanal

-
A
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

-
B
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
C
-
431-54-9
3,3,3-trifluoro-2-chloropropionyl chloride

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); chlorine In 2,4-dichloro-benzotrifluoride at 50℃; for 2.5h; Product distribution / selectivity; | A 62.5% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With sulfuric acid; water at 90℃; for 20h; | 61% |
-
-
122372-40-1
2-(2,2,2-trifluoroethylidene)-1,3-dithiane

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; mercury(II) oxide In tetrahydrofuran for 26h; Heating; | 60% |
-
-
460-92-4
1-chloro-1,1,3,3,3-pentafluoropropane

-
-
64712-27-2
1,1-dichloro-1,3,3,3-tetrafluoropropane

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-chloro-1,1,3,3,3-pentafluoropropane; 1,1-dichloro-1,3,3,3-tetrafluoropropane With sulfuric acid; sulfur trioxide at 100 - 125℃; for 19 - 30h; Stage #2: With water at 20℃; Product distribution / selectivity; | 22% |

| Conditions | Yield |
|---|---|
| With sodium dichromate; sulfuric acid |

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With water |
-
-
10472-02-3
trifluoromethyl-malonic acid dinitrile

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
354-48-3
1,1,1-tribromotrifluoroethane

-
-
124-38-9
carbon dioxide

-
A
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With water; hydrogen cation; zinc In N,N-dimethyl-formamide for 6h; irradiated by ultrasound; | A 0.1 % Spectr. B 1.0 % Spectr. |

-
-
382-21-8
perfluoroisobutylene

-
A
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
B
-
564-10-3
3,3,3-trifluoro-2-(trifluoromethyl)propanoic acid

| Conditions | Yield |
|---|---|
| With moisture; Copper(I) trifluoromethanethiolate In acetonitrile 1.) -78 deg C, 2 h, 2.) from 55 deg C to 60 deg C, 4 h; Further byproducts given. Title compound not separated from byproducts; |

-
-
382-21-8
perfluoroisobutylene

-
A
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
B
-
39902-62-0
2,2,3,3-tetra(trifluoromethyl)-perfluorobutane

| Conditions | Yield |
|---|---|
| With moisture; Copper(I) trifluoromethanethiolate In acetonitrile 1.) -78 deg C, 2 h, 2.) from 55 deg C to 60 deg C, 4 h; Further byproducts given. Title compound not separated from byproducts; |

-
-
134736-46-2
trifluoromethylketene

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With water Rate constant; |

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide In chloroform at 65℃; for 9h; | 1.7 g |
-
-
2314-97-8
iodotrifluoromethane

-
-
74786-02-0
1-(tert-butyldimethylsilyloxy)-1-tert-butoxyethylene

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With triethyl borane; oxonium 1.) hexane, RT, 2h, 2.) RT, 15 h; Yield given. Multistep reaction; |
-
-
661-54-1
3,3,3-trifluoroprop-1-yne

-
-
7664-93-9
sulfuric acid

-
A
-
75-46-7
trifluoromethan

-
B
-
421-50-1
1,1,1-trifluoro-2-propanone

-
C
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
D
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

| Conditions | Yield |
|---|---|
| at 135℃; |


| Conditions | Yield |
|---|---|
| In diethyl ether for 0.25h; Photolysis; |
-
-
116586-94-8
3,3,3-trifluoropropanal dimethyl acetal

-
A
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
B
-
18830-44-9
methyl 3,3,3-trifluoropropionate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; dihydrogen peroxide; iron(III) chloride at 80℃; for 1h; | A 95 % Chromat. B 3 % Chromat. |
-
-
116586-94-8
3,3,3-trifluoropropanal dimethyl acetal

-
A
-
460-40-2
3,3,3-trifluoropropanal

-
B
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
C
-
18830-44-9
methyl 3,3,3-trifluoropropionate

| Conditions | Yield |
|---|---|
| With copper(I) oxide; hydrogenchloride; dihydrogen peroxide at 80℃; | A 2 % Chromat. B 91 % Chromat. C 7 % Chromat. |
| With hydrogenchloride; dihydrogen peroxide; tungsten(VI) chloride at 80℃; | A 44 % Chromat. B 9 % Chromat. C 4 % Chromat. |
| With hydrogenchloride; dihydrogen peroxide at 80℃; | A 22 % Chromat. B 12 % Chromat. C 31 % Chromat. |

-
-
460-40-2
3,3,3-trifluoropropanal

-
A
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

-
B
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
C
-
19256-25-8
3,3,3-trifluoro-2-chloropropionaldehyde

-
D
-
431-54-9
3,3,3-trifluoro-2-chloropropionyl chloride

-
E
-
82107-24-2
2,2-dichloro-3,3,3-trifluoro-propionaldehyde

| Conditions | Yield |
|---|---|
| With chlorine at 50℃; for 4h; Product distribution / selectivity; | |
| With sulfuryl dichloride; dibenzoyl peroxide In water at 50℃; for 2.08333h; Product distribution / selectivity; | |
| With sulfuryl dichloride; 2,2'-azobis(isobutyronitrile) In 2,4-dichloro-benzotrifluoride at 50℃; for 2.08333h; Product distribution / selectivity; |

-
-
460-40-2
3,3,3-trifluoropropanal

-
A
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

-
B
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
C
-
19256-25-8
3,3,3-trifluoro-2-chloropropionaldehyde

-
D
-
431-54-9
3,3,3-trifluoro-2-chloropropionyl chloride

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; dibenzoyl peroxide In 2,4-dichloro-benzotrifluoride; water at 50℃; for 2.08333h; Product distribution / selectivity; | |
| With sulfuryl dichloride; 2,2'-azobis(isobutyronitrile) In 2,4-dichloro-benzotrifluoride at 50 - 65℃; for 2h; Product distribution / selectivity; | |
| With trichloroisocyanuric acid In acetonitrile at 50℃; for 4.08333h; Product distribution / selectivity; |

-
-
460-40-2
3,3,3-trifluoropropanal

-
A
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

-
B
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
C
-
19256-25-8
3,3,3-trifluoro-2-chloropropionaldehyde

-
D
-
82107-24-2
2,2-dichloro-3,3,3-trifluoro-propionaldehyde

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; 2,2'-azobis(isobutyronitrile) In acetonitrile at 50℃; for 4.08333h; Product distribution / selectivity; |

-
-
460-40-2
3,3,3-trifluoropropanal

-
A
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

-
B
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
C
-
19256-25-8
3,3,3-trifluoro-2-chloropropionaldehyde

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; 2,2'-azobis(isobutyronitrile) In toluene at 50℃; for 2.08333h; Product distribution / selectivity; |

-
-
460-40-2
3,3,3-trifluoropropanal

-
A
-
64-18-6
formic acid

-
B
-
353-50-4
Carbonyl fluoride

-
C
-
75-89-8
2,2,2-trifluoroethanol

-
D
-
75-90-1
2,2,2-Trifluoroacetaldehyde

-
E
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| Quantum yield; Mechanism; Pressure; Photolysis; UV-irradiation; Air; |

-
-
1402718-98-2
S-(3,3,3-trifluoropropionyl)-N-acetylcysteamine

-
A
-
1190-73-4
2-(acetylamino)ethanethiol

-
B
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With erythromycin thioesterase In dimethylsulfoxide-d6 pH=7.5; Kinetics; aq. buffer; Enzymatic reaction; |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 10 - 35℃; | 100% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 10 - 35℃; | 100% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In tetrahydrofuran; N,N-dimethyl-formamide at 65℃; for 24h; | 100% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
41463-83-6
3,3,3-trifluoropropanoyl chloride

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride at 65℃; Cooling with ice; | 99% |
| With thionyl chloride; N,N-dimethyl-formamide at 70℃; for 4h; | 72% |
| With Phthaloyl dichloride for 3h; Heating; | 69% |

| Conditions | Yield |
|---|---|
| at 100℃; for 24h; Sealed tube; | 98% |

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
100-51-6
benzyl alcohol

-
-
78686-91-6
benzyl 3,3,3-trifluoropropanoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide | 97% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 6h; Inert atmosphere; | 96% |
| Stage #1: 3,3,3-Trifluoropropionic acid With oxalyl dichloride at 20 - 50℃; Stage #2: benzyl alcohol for 10h; | 94% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In diethyl ether at 20℃; for 24h; | 96% |
-
-
661485-12-7
methyl 4'-{[(3-aminopyridin-2-yl)amino]methyl}-biphenyl-2-carboxylate

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride | 95% |
-
-
851901-72-9
4'-[(3-amino-4-methyl-pyridin-2-ylamino)-methyl]-biphenyl-2-carboxylic acid methyl ester

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride | 95% |
-
-
851901-73-0
4'-[(R)-1-(3-Amino-4-methyl-pyridin-2-ylamino)-ethyl]-biphenyl-2-carboxylic acid methyl ester

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride | 95% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With oxalyl dichloride at 0℃; for 0.166667h; Inert atmosphere; Stage #2: 3,3,3-Trifluoropropionic acid at 0 - 70℃; for 1.16667h; | 95% |
| With trichlorophosphate at 15 - 60℃; for 18h; Inert atmosphere; | 82% |
| With trichlorophosphate at 60℃; for 16h; | 63.7% |
| With phosgene at 0 - 70℃; for 1h; | |
| With oxalyl dichloride at 70℃; for 1h; Inert atmosphere; |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
1338323-37-7
(1R,2R,3S,5R)-2,6,6-trimethyl-2-(naphthalen-1-ylmethoxy)bicyclo[3.1.1]heptan-3-ol

-
-
1338323-42-4
(1R,2R,3S,5R)-2,6,6-trimethyl-2-(naphthalen-1-ylmethoxy)bicyclo[3.1.1]heptan-3-yl 3,3,3-trifluoropropanoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 12h; Inert atmosphere; | 95% |
-
-
4701-17-1
5-bromo-2-thiophencarboxaldehyde

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
1352743-46-4
C8H4BrF3O2S

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-thiophencarboxaldehyde; 3,3,3-Trifluoropropionic acid With titanium tetrachloride In tetrahydrofuran; dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine In tetrahydrofuran; dichloromethane at 20℃; for 40h; Knoevenagel condensation; Stage #3: With water In tetrahydrofuran; dichloromethane at 20℃; optical yield given as %de; stereoselective reaction; | 95% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| In octane; benzene at 20℃; for 24h; | 95% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
147081-44-5
(3S)-3-amino-1-(tert-butoxycarbonyl)-pyrrolidine

-
-
891944-04-0
tert-butyl (3S)-3-[(3,3,3-trifluoropropanoyl)amino]pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In dichloromethane; ethyl acetate at 0 - 20℃; for 1.5h; | 94% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 12h; | 94% |

| Conditions | Yield |
|---|---|
| In octane; benzene at 20℃; | 94% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3,3,3-Trifluoropropionic acid; 1-(4-chlorophenyl)-2,2-difluoro-2-(4-nitrophenyl)ethan-1-ol With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 0.25h; Stage #2: With dmap In dichloromethane at 20℃; for 24h; | 93% |
-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
405878-89-9
3,3,3-trifluoroacetic acid N-hydroxysuccinimide ester

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; | 92% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
62-53-3
aniline

-
-
351-12-2
3,3,3-trifluoro-N-phenylpropionamide

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 10 - 20℃; for 12h; | 92% |
| Stage #1: 3,3,3-Trifluoropropionic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 3h; Stage #2: aniline With triethylamine In dichloromethane; N,N-dimethyl-formamide at 0℃; | 92% |
| Stage #1: 3,3,3-Trifluoropropionic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 3h; Schlenk technique; Stage #2: aniline With triethylamine In dichloromethane; N,N-dimethyl-formamide at 0℃; Schlenk technique; |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
176214-18-9
N-[3-(dimethylamino)-2-(trifluoromethyl)-2-propenylidene]-N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 70℃; for 1h; | 91% |
| With trichlorophosphate at 70℃; Inert atmosphere; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,3,3-Trifluoropropionic acid; benzaldehyde With titanium tetrachloride In tetrahydrofuran; dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine In tetrahydrofuran; dichloromethane at 20℃; for 40h; Knoevenagel condensation; Stage #3: With water In tetrahydrofuran; dichloromethane at 20℃; optical yield given as %de; stereoselective reaction; | 91% |
| Stage #1: 3,3,3-Trifluoropropionic acid; benzaldehyde With titanium tetrachloride In tetrahydrofuran; dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine In tetrahydrofuran; dichloromethane for 40h; | 70% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
1392849-00-1
C11H7F3O3

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In acetic acid butyl ester; ethyl acetate at 120℃; Perkin condensation; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,3,3-Trifluoropropionic acid; 4-fluorobenzaldehyde With titanium tetrachloride In tetrahydrofuran; dichloromethane at 20℃; for 0.5h; Aldol Condensation; Stage #2: With triethylamine In tetrahydrofuran; dichloromethane at 20℃; for 24h; | 91% |

| Conditions | Yield |
|---|---|
| In octane; benzene at 20℃; for 24h; | 91% |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
1321619-38-8
(Z)-3-(4-bromophenyl)-2-(trifluoromethyl)acrylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3,3,3-Trifluoropropionic acid; 4-bromo-benzaldehyde With titanium tetrachloride In tetrahydrofuran; dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine In tetrahydrofuran; dichloromethane at 20℃; for 40h; Knoevenagel condensation; Stage #3: With water In tetrahydrofuran; dichloromethane at 20℃; optical yield given as %de; stereoselective reaction; | 90% |
| Stage #1: 3,3,3-Trifluoropropionic acid; 4-bromo-benzaldehyde With titanium tetrachloride In tetrahydrofuran; dichloromethane at -10℃; for 0.5h; Stage #2: With 4-methyl-morpholine In tetrahydrofuran; dichloromethane at 20℃; for 40h; Reagent/catalyst; Solvent; Temperature; regioselective reaction; | 69% |
| Stage #1: 3,3,3-Trifluoropropionic acid; 4-bromo-benzaldehyde With titanium tetrachloride In tetrahydrofuran; dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine In tetrahydrofuran; dichloromethane for 40h; | 26% |
| Stage #1: 3,3,3-Trifluoropropionic acid; 4-bromo-benzaldehyde With titanium tetrachloride In tetrahydrofuran; dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine for 40h; |
-
-
2516-99-6
3,3,3-Trifluoropropionic acid

-
-
57946-56-2
2-fluoro-4-chloroaniline

-
-
1538626-78-6
N-(4-chloro-2-fluoro-phenyl)-3,3,3-trifluoro-propionamide

| Conditions | Yield |
|---|---|
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane | 90% |
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane | 90% |
| With triethylamine; HATU In dichloromethane |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 10 - 20℃; for 12h; | 89% |
3,3,3-Trifluoropropionic acid Chemical Properties
IUPAC Name: 3,3,3-Trifluoropropanoate
CAS: 2516-99-6
Molecular Formula: C3H3F3O2
Molecular Weight: 128.05
Molecular structure:
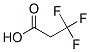
Melting point: 9.7 °C(lit.)
BRN: 1751796
ACD/LogD (pH 5.5): -1.31
ACD/LogD (pH 7.4): -2.61
ACD/BCF (pH 5.5): 1
ACD/BCF (pH 7.4): 1
ACD/KOC (pH 5.5): 1
ACD/KOC (pH 7.4): 1
H bond acceptors: 2
H bond donors: 1
Freely Rotating Bonds: 1
Index of Refraction: 1.325
Molar Refractivity: 17.96 cm3
Molar Volume: 89 cm3
Polarizability: 7.12 10-24cm3
Surface Tension: 23.8 dyne/cm
Density: 1.437 g/cm3
Flash Point: 31.2 °C
Enthalpy of Vaporization: 40.36 kJ/mol
Boiling Point: 128 °C at 760 mmHg
Vapour Pressure: 6.63 mmHg at 25°C
Product Categories: Fluorochemicals; C1 to C5; Carbonyl Compounds; Carboxylic Acids
3,3,3-Trifluoropropionic acid Safety Profile
Hazard Codes: C![]()
Risk Statements: 34 (34: Causes burns)
Safety Statements: 26-36/37/39-45 (26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice 36/37/39: Wear suitable protective clothing, gloves and eye/face protection 45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible))
RIDADR: UN 3265 8/PG 2
WGK Germany: 3
Hazard Note: Corrosive
HazardClass: 8
PackingGroup: II
3,3,3-Trifluoropropionic acid Specification
3,3,3-Trifluoropropionic acid , with CAS number of 2516-99-6, can be called 3,3,3-Trifluoropropionic acid 98% ; 3,3,3-Trifluoropropanoicacid ; Trifluoromethylacetic acid ; propanoic acid, 3,3,3-trifluoro- . It is usually colorless clear liquid. 3,3,3-Trifluoropropionic acid (CAS NO.2516-99-6) is commonly used as pharmaceutical intermediate.
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 2517-04-6
- 25173-37-9
- 25173-68-6
- 25173-72-2
- 25173-76-6
- 2517-43-3
- 25177-85-9
- 25178-38-5
- 2518-24-3
- 25182-74-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View