-
Name
3-Aminobenzylamine
- EINECS 412-230-2
- CAS No. 4403-70-7
- Article Data26
- CAS DataBase
- Density 1.093 g/cm3
- Solubility
- Melting Point 43 °C
- Formula C7H10N2
- Boiling Point 267.6 °C at 760 mmHg
- Molecular Weight 122.17
- Flash Point 136 °C
- Transport Information UN 3259 8/PG 3
- Appearance Pale brown low melting solid
- Safety 26-36/37/39-61-45-22
- Risk Codes 34-51/53-22
-
Molecular Structure
-
Hazard Symbols
 N,
N,  C
C
- Synonyms Toluene-a,3-diamine (7CI,8CI);3-(Aminomethyl)aniline;3-Aminobenzenemethanamine;3-Aminomethylphenylamine;m-Aminobenzylamine;AC1Q53VN;TL8003076;
- PSA 52.04000
- LogP 2.00900
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol | 100% |
| With C28H29Cl2CoNP2; hydrogen; sodium triethylborohydride In 1,4-dioxane at 80℃; under 37503.8 Torr; for 6h; | 75% |
| In methanol; palladium-carbon | 0.516 g (~100%) |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate at 20℃; for 0.0333333h; neat (no solvent, solid phase); | 95% |
| With borohydride exchange resin; nickel diacetate In methanol at 25℃; for 10h; | 82% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; copper ferrite In water for 0.333333h; Reflux; Green chemistry; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| Rh-C | 89.2% |
| With sodium hydroxide; hydrogen; toluene-4-sulfonic acid; Pt-C In 1,4-dioxane | 72.9% |

| Conditions | Yield |
|---|---|
| With hydrogen; Pd-C In acetic acid; ethyl acetate | 77% |
-
-
126799-84-6
1-azidomethyl-3-nitrobenzene

-
-
4403-70-7
m-aminobenzylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; indium In tetrahydrofuran at 20℃; for 5h; | 73% |

| Conditions | Yield |
|---|---|
| With pyridine; carbon palladium In methanol; ethyl acetate | 53% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin |
-
-
77147-14-9
2-(3-aminobenzyl)isoindoline-1,3-dione

-
-
4403-70-7
m-aminobenzylamine

| Conditions | Yield |
|---|---|
| With ethanol; hydrazine hydrate Erwaermen des Reaktionsprodukts mit wss.Salzsaeure; |
-
-
26177-43-5
3-nitrobenzylamine hydrochloride

-
-
4403-70-7
m-aminobenzylamine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol for 1h; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran |
-
-
21081-63-0
2-(3-nitrobenzyl)isoindoline-1,3-dione

-
-
4403-70-7
m-aminobenzylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: platinum; acetic acid / Hydrogenation 2: ethanol; hydrazine hydrate / Erwaermen des Reaktionsprodukts mit wss.Salzsaeure View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 120 °C / und Spaltung des entstandenen N-<3-nitro-benzyl>-phthalimids durch Erhitzen mit rauch. Salzsaeure bei 200grad 2: tin; hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; thionyl chloride; sodium hydrogencarbonate; methylamine; palladium-carbon In N-methyl-acetamide; methanol; chloroform; water |

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
4403-70-7
m-aminobenzylamine

-
-
147291-66-5
3-tert-butoxycarbonylaminomethylaniline

| Conditions | Yield |
|---|---|
| In 1,4-dioxane | 100% |
| In acetonitrile at 20℃; | 100% |
| With TEA In dichloromethane at 0℃; for 2h; | 100% |
-
-
383-63-1
ethyl trifluoroacetate,

-
-
4403-70-7
m-aminobenzylamine

-
-
400720-32-3
N-(3-aminobenzyl)-2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |
| In tetrahydrofuran at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; trimethyl orthoformate for 1h; Reflux; Stage #2: m-aminobenzylamine for 2h; Reflux; | 100% |

-
-
4403-70-7
m-aminobenzylamine

-
-
250684-60-7
(3-aminobenzyl)carbamic acid 9H-fluoren-9-ylmethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 3.5h; pH=8; | 99% |
-
-
65191-07-3
ethyl 5-methyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidine-2-carboxylate

-
-
4403-70-7
m-aminobenzylamine

-
-
935760-31-9
N-[(3-aminophenyl)methyl]-5-methyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidine-2-carboxamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 80℃; for 15h; | 97% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
4403-70-7
m-aminobenzylamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; acetonitrile for 1h; | 94% |

| Conditions | Yield |
|---|---|
| With acetic acid at 80℃; for 20h; Sealed tube; chemoselective reaction; | 94% |
-
-
4403-70-7
m-aminobenzylamine

-
-
501-53-1
benzyl chloroformate

-
-
1095512-62-1
3-(aminomethyl)-N-(benzyloxycarbonyl)phenylamine

| Conditions | Yield |
|---|---|
| With acetic acid In 1,4-dioxane; water at 20℃; pH=4.5; regioselective reaction; | 93% |
-
-
614-45-9
tert-Butyl peroxybenzoate

-
-
4403-70-7
m-aminobenzylamine

-
-
180150-44-1
N-(3-aminobenzyl)benzamide

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 14h; chemoselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With carbon dioxide In tetrahydrofuran at 20℃; under 760.051 Torr; for 24h; UV-irradiation; | 93% |
| With carbon dioxide In tetrahydrofuran at 25℃; for 24h; UV-irradiation; | 93% |

| Conditions | Yield |
|---|---|
| With C68H64Cl2N6P2Ru2(4+)*2F6P(1-)*2Cl(1-); caesium carbonate In N,N-dimethyl-formamide at 100℃; for 24h; Inert atmosphere; Green chemistry; | 92.8% |
| With HRu(1,3-bis(6'-methyl-2'-pyridylimino)isoindoline)(PPh3)2 In toluene at 110℃; for 24h; Inert atmosphere; Glovebox; chemoselective reaction; | 54% |
-
-
4403-70-7
m-aminobenzylamine

-
-
13790-39-1
6,7-dimethoxy-4-chloroquinazoline

| Conditions | Yield |
|---|---|
| With triethylamine In isopropyl alcohol at 80℃; for 12h; | 92.56% |
| With triethylamine In dichloromethane at 20℃; for 8h; | |
| With triethylamine In dichloromethane at 20℃; |
-
-
14542-93-9
1,1,3,3-tetramethylbutane isonitrile

-
-
4403-70-7
m-aminobenzylamine

| Conditions | Yield |
|---|---|
| With sulfur at 20℃; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 20℃; for 5h; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With iron oxide In ethanol at 25℃; for 2h; | 91% |
| With manganese oxide octahedral molecular sieve-supported copper hydroxide; air In ethanol at 20℃; for 2h; Green chemistry; | 70% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 0.166667h; | 91% |

-
-
4403-70-7
m-aminobenzylamine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
A
-
1321455-62-2
N-(3-(aminomethyl)phenyl)-1-tert-butyl-1,1-dimethylsilanamine

-
B
-
1321455-64-4
1-tert-butyl-N-(3-(tert-butyldimethylsilylamino)benzyl)-1,1-dimethylsilanamine

| Conditions | Yield |
|---|---|
| Stage #1: m-aminobenzylamine With methyllithium In 2-methyltetrahydrofuran; diethyl ether at -50℃; Inert atmosphere; Stage #2: tert-butyldimethylsilyl chloride In 2-methyltetrahydrofuran; diethyl ether at -50℃; for 3.16667h; Inert atmosphere; regioselective reaction; | A 90% B 4% |


| Conditions | Yield |
|---|---|
| With rhodium(III) chloride trihydrate In toluene at 100℃; under 37503.8 Torr; for 20h; Inert atmosphere; Autoclave; | 90% |


| Conditions | Yield |
|---|---|
| With 1,1,3,3-Tetramethyldisiloxane; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 25℃; for 24h; Sealed tube; | 89% |
-
-
4403-70-7
m-aminobenzylamine

-
-
97087-59-7
α-(3-aminocyclohexyl)methylamine

| Conditions | Yield |
|---|---|
| 87% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With dmap; diisopropyl-carbodiimide In dichloromethane at 20℃; for 2h; Inert atmosphere; | 86.2% |

| Conditions | Yield |
|---|---|
| With benzoic acid In para-xylene at 130℃; for 8h; Inert atmosphere; Schlenk technique; | 86% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
4403-70-7
m-aminobenzylamine

-
-
7338-94-5
1,3-diphenyl-2-propynone

| Conditions | Yield |
|---|---|
| Stage #1: m-aminobenzylamine; 1,3-diphenyl-2-propynone With lithium tert-butoxide In dichloromethane at 20℃; for 2h; Stage #2: di-tert-butyl dicarbonate In dichloromethane at 20℃; for 12h; chemoselective reaction; | 86% |

3-Aminobenzylamine Specification
The chemical with CAS registry number of 4403-70-7 is also known as 3-Aminobenzylamine. The IUPAC name is 3-(Aminomethyl)aniline. It belongs to product categories of Anilines, Aromatic Amines and Nitro Compounds; Amine; Amines. Its EINECS registry number is 412-230-2. In addition, the formula is C7H10N2 and the molecular weight is 122.17. This chemical is a pale brown low melting solid and should be sealed in ventilated and dry place without light.
Physical properties about 3-Aminobenzylamine are: (1)ACD/LogP: -0.19; (2)ACD/LogD (pH 5.5): -3.26; (3)ACD/LogD (pH 7.4): -2.33; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 4; (10)#Freely Rotating Bonds: 3; (11)Index of Refraction: 1.614; (12)Molar Refractivity: 38.94 cm3; (13)Molar Volume: 111.7 cm3; (14)Surface Tension: 51.3 dyne/cm; (15)Density: 1.093 g/cm3; (16)Flash Point: 136 °C; (17)Enthalpy of Vaporization: 50.56 kJ/mol; (18)Boiling Point: 267.6 °C at 760 mmHg; (19)Vapour Pressure: 0.0081 mmHg at 25 °C.
Preparation of 3-Aminobenzylamine: it is prepared by reaction of 3-nitro-benzaldehyde oxime. The reaction needs reagents borohydride exchange resin, Ni(OAc)2 and solvent methanol at the temperature of 25 °C for 10 hours. The yield is about 82%.

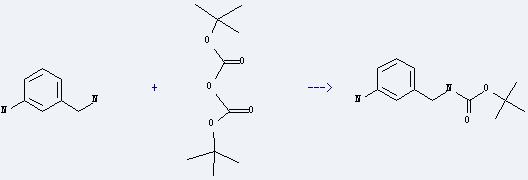
Uses of 3-Aminobenzylamine: it is used to produce (3-aminobenzyl)carbamic acid tert-butyl ester by reaction with di-tert-butyl dicarbonate. The reaction occurs with reagent dioxane and the yield is about 100%.
When you are using this chemical, please be cautious about it. As a chemical, it is harmful if swallowed and may cause burns. What's more, it is toxic to aquatic organisms that may cause long-term adverse effects in the aquatic environment. During using it, wear suitable protective clothing, gloves and eye/face protection. Do not breathe dust and avoid release to the environment. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC(=CC(=C1)N)CN
2. InChI: InChI=1S/C7H10N2/c8-5-6-2-1-3-7(9)4-6/h1-4H,5,8-9H2
3. InChIKey: ZDBWYUOUYNQZBM-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View