-
Name
3-Hydroxyadamantane-1-carboxylic acid
- EINECS
- CAS No. 42711-75-1
- Article Data34
- CAS DataBase
- Density 1.419 g/cm3
- Solubility
- Melting Point 203 °C
- Formula C11H16O3
- Boiling Point 357.2 °C at 760 mmHg
- Molecular Weight 196.246
- Flash Point 184 °C
- Transport Information
- Appearance
- Safety 26-36/37/39-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1-Adamantanecarboxylicacid, 3-hydroxy- (7CI);1-Carboxy-3-adamantanol;3-Carboxy-1-hydroxyadamantane;Tricyclo[3.3.1.13,7]decane-1-carboxylicacid, 3-hydroxy-;
- PSA 57.53000
- LogP 1.40230
Synthetic route
-
-
828-51-3
1-Adamantanecarboxylic acid

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 0℃; | 98% |
| Stage #1: 1-Adamantanecarboxylic acid With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water; tert-butyl alcohol at 40℃; for 0.5h; Green chemistry; Stage #2: With potassium permanganate In water; tert-butyl alcohol at 60℃; for 8.5h; Green chemistry; | 94.12% |
| With nitric acid at 10 - 35℃; for 3.5h; Time; | 92.6% |

-
-
828-51-3
1-Adamantanecarboxylic acid

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With acetic acid | 80% |
-
-
770-71-8
1-adamantanemethanol

-
A
-
38584-37-1
1-hydroxy-3-hydroxymethyladamantane

-
B
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-adamantanemethanol With nitric acid at 20℃; for 1h; Stage #2: With hydrazine hydrate In butan-1-ol for 3h; Reflux; | A 75% B 8% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-adamantandiol With sulfuric acid at 35℃; for 0.5h; Stage #2: formic acid at 20 - 40℃; for 3h; | 38% |
-
-
828-51-3
1-Adamantanecarboxylic acid

-
A
-
56674-87-4
4-oxoadamantane-1-carboxylic acid

-
B
-
81968-77-6
4-hydroxyadamantane-1-carboxylic acid

-
C
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; phosphate buffer; oxygen; iron(II) sulfate In water at 40℃; for 3h; Product distribution; investigation of the oxy-functionalization of adamantane-1-carboxylic acid; | A 12% B 5% C 34% |

-
-
21816-08-0
3-bromoadamantane-1-carboxylic acid

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With silver nitrate In 1,4-dioxane |
-
-
828-51-3
1-Adamantanecarboxylic acid

-
A
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
B
-
880-50-2
3-fluoro-1-adamantane-carboxylic acid

| Conditions | Yield |
|---|---|
| With difluoroether |
-
-
68435-07-4
1-methoxycarbonyl-3-hydroxyadamantane

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol |

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; water; triethylamine solvolysis; |
-
-
768-90-1
1-Adamantyl bromide

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sulfuric acid / 20 °C 1.2: 4.5 h / 10 - 35 °C 2.1: nitric acid / 5.5 h / 10 - 35 °C View Scheme |
-
-
828-51-3
1-Adamantanecarboxylic acid

-
A
-
56674-87-4
4-oxoadamantane-1-carboxylic acid

-
B
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; C60H66N18*3Fe(2+)*3O4S(2-) In water at 60℃; for 24h; | A 6 %Chromat. B 26 %Chromat. |
-
-
64-18-6
formic acid

-
-
5001-18-3
1,3-adamantandiol

-
A
-
828-51-3
1-Adamantanecarboxylic acid

-
B
-
39269-10-8
adamantane-1,3-dicarboxylic acid

-
C
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-adamantandiol With sulfuric acid at 35℃; for 0.5h; Stage #2: formic acid at 20 - 40℃; for 3h; |

-
-
768-95-6
1-adamanthanol

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid / 3.25 h / 10 - 35 °C 2: nitric acid / 3.5 h / 10 - 35 °C View Scheme |
-
-
50795-82-9
3-nitroxyadamantane-1-carboxylic acid

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With acetic acid; urea In water at 25 - 90℃; for 5h; |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 50℃; Friedel-Crafts Alkylation; | 100% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
42711-77-3
3-iodoadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogen iodide at 60℃; for 16h; | 99.8% |
| With phosphorus pentoxide; phosphoric acid; potassium iodide at 120℃; for 3h; Inert atmosphere; | 95% |
| With phosphoric acid; phosphorus pentoxide; potassium iodide |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
21816-08-0
3-bromoadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide at 90℃; for 3.5h; | 99.8% |
| With hydrogen bromide In water at 90℃; for 4.5h; | 92% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
74-88-4
methyl iodide

-
-
68435-07-4
1-methoxycarbonyl-3-hydroxyadamantane

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydrogencarbonate In acetone at 20℃; for 48h; | 99% |
-
-
1221151-98-9
2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl ortho-(hex-1-ynyl)benzoate

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With trifluoromethanesulfonyloxy(triphenylphosphine)gold(I); boron trifluoride diethyl etherate; 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; Molecular sieve; Inert atmosphere; chemoselective reaction; | 97% |
-
-
67-56-1
methanol

-
-
828-51-3
1-Adamantanecarboxylic acid

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
A
-
711-01-3
methyl adamantane-1-carboxylate

-
B
-
68435-07-4
1-methoxycarbonyl-3-hydroxyadamantane

| Conditions | Yield |
|---|---|
| With sulfuric acid Reflux; | A 97% B n/a |

-
-
67-56-1
methanol

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
68435-07-4
1-methoxycarbonyl-3-hydroxyadamantane

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,2-dichloro-ethane for 20h; Heating; | 96% |
| With sulfuric acid for 12h; Inert atmosphere; Reflux; |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
182496-69-1
25,26,27,28-tetrahydroxy-2,8,14,20-tetrathiacalix[4]arene

| Conditions | Yield |
|---|---|
| trifluorormethanesulfonic acid In trifluoroacetic acid for 24h; Heating; | 96% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
248590-47-8
25,26,27,28-tetrakis(hydroxy)calix[4]arene

-
-
765943-01-9
p-(3-carboxy-1-adamantyl)calix[4]arene

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; trifluoroacetic acid In 1,2-dichloro-ethane at 85 - 90℃; for 12h; | 96% |
| With trifluorormethanesulfonic acid; trifluoroacetic acid In 1,2-dichloro-ethane at 60 - 65℃; for 10h; |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
38584-37-1
1-hydroxy-3-hydroxymethyladamantane

| Conditions | Yield |
|---|---|
| 95% | |
| Stage #1: 3-hydroxyadamantane-1-carboxylic acid With sodium tetrahydroborate In tetrahydrofuran at 0 - 31℃; for 2.5h; Stage #2: With boron trifluoride diethyl etherate In tetrahydrofuran at 0 - 30℃; for 14.5h; Stage #3: With water In tetrahydrofuran at 0 - 10℃; for 1h; | 29.5 g |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
40556-45-4
1-fluoro-3-(trifluoromethyl)adamantane

| Conditions | Yield |
|---|---|
| With sulfur tetrafluoride; water at -178 - 40℃; for 24h; Autoclave; | 95% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
71-36-3
butan-1-ol

-
-
247029-06-7
n-butyl 3-hydroxy-1-adamantanecarboxylate

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene for 5h; Heating / reflux; | 94.1% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
67-68-5
dimethyl sulfoxide

| Conditions | Yield |
|---|---|
| With triethylamine at 160℃; under 760.051 Torr; for 20h; Pummerer Sulfoxide Rearrangement; Schlenk technique; Inert atmosphere; Green chemistry; | 94% |
-
-
868-77-9
2-methyl-2-propenoic acid 2-hydroxyethyl ester

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 1,2-dichloro-ethane at 12 - 20℃; for 22h; Temperature; Reagent/catalyst; Concentration; | 93.9% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
71-43-2
benzene

-
-
113392-33-9
1,4-di(3-carboxy-1-adamantyl)benzene

| Conditions | Yield |
|---|---|
| With sulfuric acid at 15 - 20℃; for 4h; | 93% |

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; sodium hydride In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 91% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0 - 20℃; for 18h; Inert atmosphere; | 91% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
814-68-6
acryloyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; Cooling with ice; | 90.6% |

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
38584-37-1
1-hydroxy-3-hydroxymethyladamantane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 90% |
-
-
923-26-2
2-hydroxypropyl methacrylate

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 1,2-dichloro-ethane at 12 - 20℃; for 22h; Temperature; Reagent/catalyst; Solvent; Concentration; | 89.8% |
-
-
95-16-9
1,3-Benzothiazole

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver nitrate In dichloromethane; water at 20℃; for 8h; | 89% |

| Conditions | Yield |
|---|---|
| With [Co(dyimethylglyoximate(1-))2(pyridine)2]PF6; tetrabutylammonium acetate In ethyl acetate at 40 - 44℃; for 30h; Irradiation; Schlenk technique; Inert atmosphere; | 88% |
-
-
1230076-22-8
2,3,4-tri-O-acetyl-L-rhamnopyranosyl ortho-hexynylbenzoate

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With trifluoromethanesulfonyloxy(triphenylphosphine)gold(I); boron trifluoride diethyl etherate; 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; Molecular sieve; Inert atmosphere; chemoselective reaction; | 87% |
-
-
1584705-82-7
(((2-(2-iodophenyl)propan-2-yl)oxy)(trifluoromethyl)sulfane)

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
1624333-35-2
(1r,3s,5R,7S)-3-((trifluoromethyl)thio)adamantan-1-ol

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; sodium dodecyl sulfate; silver nitrate In water at 50℃; for 12h; Inert atmosphere; | 86% |
-
-
106-42-3
para-xylene

-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
120383-96-2
1,4-di(3-carboxy-1-adamantyl)-2,5-dimethylbenzene

| Conditions | Yield |
|---|---|
| With sulfuric acid at 15 - 20℃; for 4h; | 85% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; copper; silver nitrate In water; acetonitrile at 110℃; for 12h; | 85% |
-
-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-
-
107-30-2
chloromethyl methyl ether

| Conditions | Yield |
|---|---|
| With triethylamine at 10℃; for 1h; Temperature; Inert atmosphere; | 85% |
3-Hydroxyadamantane-1-carboxylic acid Chemical Properties
Molecule structure of 3-Hydroxyadamantane-1-carboxylic acid (CAS NO.42711-75-1):
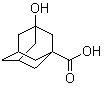
IUPAC Name: (5S,7R)-3-Hydroxyadamantane-1-carboxylate
Molecular Weight: 196.24 g/mol
Molecular Formula: C11H16O3
Melting Point: 203 °C
Index of Refraction: 1.638
Molar Refractivity: 49.72 cm3
Molar Volume: 138.2 cm3
Surface Tension: 77.2 dyne/cm
Density: 1.419 g/cm3
Flash Point: 184 °C
Enthalpy of Vaporization: 69.75 kJ/mol
Boiling Point: 357.2 °C at 760 mmHg
Vapour Pressure: 1.55E-06 mmHg at 25 °C
SMILES: O=C(O)C13CC2CC(CC(O)(C1)C2)C3
InChI: InChI=1/C11H16O3/c12-9(13)10-2-7-1-8(3-10)5-11(14,4-7)6-10/h7-8,14H,1-6H2,(H,12,13)
InChIKey: CJJMAWPEZKYJAP-UHFFFAOYAF
Product Categories of 3-Hydroxyadamantane-1-carboxylic acid (CAS NO.42711-75-1: Adamantane derivatives;Carboxylic Acids;Carboxylic Acids;Ring Systems
3-Hydroxyadamantane-1-carboxylic acid Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36/37/39-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S37/39:Wear suitable gloves and eye/face protection.
Hazard Note: Irritant
HazardClass: IRRITANT
3-Hydroxyadamantane-1-carboxylic acid Specification
3-Hydroxyadamantane-1-carboxylic acid (CAS NO.42711-75-1) is also named as 1-carboxy-3-hydroxyadamantane ; 3-hydroxytricyclo[3.3.1.1~3,7~]decane-1-carboxylic acid ; tricyclo[3.3.1.1~3,7~]decane-1-carboxylic acid, 3-hydroxy- ; 1-Carboxy-3-adamantanol ; 3-Carboxy-1-hydroxyadamantane ; 3-hydroxy-1-adamantanecarboxylic acid ; 3-Hydroxy-adamantane-1-carboxylic acid ; 3-hydroxyadamantanecarboxylic acid ; 3-Hydroxytricyclo[3.3.1.13,7]decane-1-carboxylic acid .
Related Products
- 3-Hydroxyadamantane-1-carboxylic acid
- 42711-76-2
- 42711-77-3
- 42712-64-1
- 4271-28-7
- 4271-30-1
- 42716-73-4
- 4271-90-3
- 4271-96-9
- 42719-70-0
- 4271-99-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View