-
Name
4'-Demethylepipodophyllotoxin
- EINECS 613-823-0
- CAS No. 6559-91-7
- Article Data36
- CAS DataBase
- Density 1.446 g/cm3
- Solubility
- Melting Point 246-248 °C
- Formula C21H20O8
- Boiling Point 626.5 °C at 760 mmHg
- Molecular Weight 400.385
- Flash Point 224.4 °C
- Transport Information
- Appearance White crystalline powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Epipodophyllotoxin,4'-demethyl- (7CI,8CI);Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one,5,8,8a,9-tetrahydro-9-hydroxy-5-(4-hydroxy-3,5-dimethoxyphenyl)-, [5R-(5a,5ab,8aa,9b)]-;(-)-4'-Demethylepipodophyllotoxin;4'-Demethylepipodophyllotoxin;4'-O-demethylepipodophyllotoxin;
- PSA 103.68000
- LogP 2.10620
Synthetic route

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; DL-methionine; trifluoroacetic acid at 40℃; for 0.75h; | 93% |
| Stage #1: podofilox With methanesulfonic acid; sodium iodide In dichloromethane at 0 - 20℃; for 5h; Stage #2: With barium carbonate In water; acetone at 40℃; for 0.5h; | 90% |
| Stage #1: podofilox With 1-(Trimethylsilyl)imidazole In dichloromethane at 0℃; Stage #2: With water; barium carbonate In acetone | 79% |
-
-
156350-44-6
Carbonic acid benzyl ester 4-((5R,5aR,8aR)-6,9-dioxo-5,5a,6,8,8a,9-hexahydro-furo[3',4':6,7]naphtho[2,3-d][1,3]dioxol-5-yl)-2,6-dimethoxy-phenyl ester

-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| With ruthenium(II) chloride; (S)-N2,N2'-bis(pyridin-2-ylmethyl-1,1'-binaphthyl-2,2'-diamine); hydrogen In isopropyl alcohol at 20 - 25℃; under 38002.6 Torr; for 18h; | 89% |
-
-
16477-16-0
4'-O-demethyl-4β-bromo-4-desoxypodophyllotoxin

-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| With water; barium carbonate In acetone at 40℃; for 4h; | 71% |
| With barium carbonate In water; acetone for 1h; Heating; | 52% |
-
-
64-19-7
acetic acid

-
-
518-28-5
podofilox

-
A
-
6559-91-7
4'-demethylepipodophyllotoxin

-
B
-
102306-96-7
4'-demethyl-β-apopicropodophyllotoxin

-
C
-
102306-95-6
4'-demethyl-1-O-ethyl-1-epipodophyllotoxin

-
D
-
4375-07-9
epipodophyllotoxin

| Conditions | Yield |
|---|---|
| With hydrogen bromide 1.) 15 min, 2.) RT, 20 h; Multistep reaction. Further byproducts given; |

-
-
64-19-7
acetic acid

-
-
518-28-5
podofilox

-
A
-
6559-91-7
4'-demethylepipodophyllotoxin

-
B
-
102306-96-7
4'-demethyl-β-apopicropodophyllotoxin

-
C
-
102306-97-8
3',4'-didemethylepipodophyllotoxin

-
D
-
4375-07-9
epipodophyllotoxin

| Conditions | Yield |
|---|---|
| With hydrogen bromide 1.) 15 min, 2.) RT, 20 h; Multistep reaction. Further byproducts given; |

-
-
33419-42-0
etoposide

-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium iodide; chloro-trimethyl-silane / acetonitrile / 0 °C 2: water; barium carbonate / acetone / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: sodium hydroxide / tetrahydrofuran / 25 - 30 °C 2.1: lithium diisopropyl amide / tetrahydrofuran / 3 h / -20 - -15 °C / Inert atmosphere 3.1: iron(III) chloride / dichloromethane / 1 h / 18 - 22 °C 4.1: triethylamine / dichloromethane / 1 h / -2 - 2 °C 5.1: palladium diacetate; triphenylphosphine; potassium carbonate / acetonitrile / 20 h / 75 - 85 °C 6.1: pyridine; ozone / dichloromethane; methanol / 5 h / -25 - -15 °C 6.2: 4 h / 20 °C / Inert atmosphere 7.1: (S)-N2,N2'-bis(pyridin-2-ylmethyl-1,1'-binaphthyl-2,2'-diamine); ruthenium(II) chloride; hydrogen / isopropyl alcohol / 18 h / 20 - 25 °C / 38002.6 Torr View Scheme |

-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: lithium diisopropyl amide / tetrahydrofuran / 3 h / -20 - -15 °C / Inert atmosphere 2.1: iron(III) chloride / dichloromethane / 1 h / 18 - 22 °C 3.1: triethylamine / dichloromethane / 1 h / -2 - 2 °C 4.1: palladium diacetate; triphenylphosphine; potassium carbonate / acetonitrile / 20 h / 75 - 85 °C 5.1: pyridine; ozone / dichloromethane; methanol / 5 h / -25 - -15 °C 5.2: 4 h / 20 °C / Inert atmosphere 6.1: (S)-N2,N2'-bis(pyridin-2-ylmethyl-1,1'-binaphthyl-2,2'-diamine); ruthenium(II) chloride; hydrogen / isopropyl alcohol / 18 h / 20 - 25 °C / 38002.6 Torr View Scheme |

-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: iron(III) chloride / dichloromethane / 1 h / 18 - 22 °C 2.1: triethylamine / dichloromethane / 1 h / -2 - 2 °C 3.1: palladium diacetate; triphenylphosphine; potassium carbonate / acetonitrile / 20 h / 75 - 85 °C 4.1: pyridine; ozone / dichloromethane; methanol / 5 h / -25 - -15 °C 4.2: 4 h / 20 °C / Inert atmosphere 5.1: (S)-N2,N2'-bis(pyridin-2-ylmethyl-1,1'-binaphthyl-2,2'-diamine); ruthenium(II) chloride; hydrogen / isopropyl alcohol / 18 h / 20 - 25 °C / 38002.6 Torr View Scheme |

-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / dichloromethane / 1 h / -2 - 2 °C 2.1: palladium diacetate; triphenylphosphine; potassium carbonate / acetonitrile / 20 h / 75 - 85 °C 3.1: pyridine; ozone / dichloromethane; methanol / 5 h / -25 - -15 °C 3.2: 4 h / 20 °C / Inert atmosphere 4.1: (S)-N2,N2'-bis(pyridin-2-ylmethyl-1,1'-binaphthyl-2,2'-diamine); ruthenium(II) chloride; hydrogen / isopropyl alcohol / 18 h / 20 - 25 °C / 38002.6 Torr View Scheme |

-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: palladium diacetate; triphenylphosphine; potassium carbonate / acetonitrile / 20 h / 75 - 85 °C 2.1: pyridine; ozone / dichloromethane; methanol / 5 h / -25 - -15 °C 2.2: 4 h / 20 °C / Inert atmosphere 3.1: (S)-N2,N2'-bis(pyridin-2-ylmethyl-1,1'-binaphthyl-2,2'-diamine); ruthenium(II) chloride; hydrogen / isopropyl alcohol / 18 h / 20 - 25 °C / 38002.6 Torr View Scheme |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
117604-05-4
4β-azido-4-deoxy-4′-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| With sodium azide; trifluoroacetic acid In chloroform at 20℃; | 100% |
| With sodium azide; trifluoroacetic acid In chloroform for 0.25h; | 94% |
| With sodium azide; trifluoroacetic acid In chloroform at 20℃; Cooling with ice; | 91.8% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
153230-70-7
4,4'-bis-O-(dimethyl-tert-butylsilyl)-4'-O-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 20h; | 98% |
| With 2,6-dimethylpyridine In dichloromethane at 0℃; for 1h; | 81% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
64-19-7
acetic acid

-
-
22823-30-9
4'-demethylepipodophyllotoxin diacetate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 98% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
501-53-1
benzyl chloroformate

-
-
23363-33-9
4'-demethyl-4'-O-(benzoyloxycarbonyl)epipodophyllotoxin

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 2h; | 97% |
| With triethylamine In dichloromethane at 0 - 35℃; for 2h; | 97% |
| With triethylamine In dichloromethane at 0 - 35℃; for 2h; | 97% |

| Conditions | Yield |
|---|---|
| sulfuric acid at 20℃; for 3h; Ritter Reaction; | 96% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
107-14-2
chloroacetonitrile

-
-
150059-96-4
4-chloroacetamido-4-deoxy-4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| With sulfuric acid In isopropyl alcohol at 20℃; for 1h; Inert atmosphere; | 96% |
| With sulfuric acid at 20℃; for 1h; Ritter Reaction; | 93% |
| sulfuric acid at 20℃; | 93% |
| With sulfuric acid Ritter Amidation; | |
| Ritter Amidation; |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 94% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 94% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; | 93% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 92% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 92% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 92% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
A
-
117604-05-4
4β-azido-4-deoxy-4′-demethylepipodophyllotoxin

-
B
-
117507-85-4
4β-azido-4'-dimethyl-4-deoxy-epipodophyllotoxin

| Conditions | Yield |
|---|---|
| With tris-(2-chloro-ethyl)-amine; boron trifluoride diethyl etherate In dichloromethane; benzene at -15℃; for 1h; | A 91% B n/a |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
17176-77-1
Dibenzyl phosphite

| Conditions | Yield |
|---|---|
| With tetrachloromethane; dmap; N-ethyl-N,N-diisopropylamine In acetonitrile at -10℃; | 90% |
| With dmap; N-ethyl-N,N-diisopropylamine In tetrachloromethane; acetonitrile at -10℃; Inert atmosphere; | 90.1% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
102306-96-7
4'-demethyl-β-apopicropodophyllotoxin

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic anhydride for 1h; Heating; | 90% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
118356-07-3
4'-O-tert-butyldimethylsilanyl-4'-O-demethyl-4-epipodophyllotoxin

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 1.5h; | 89% |
| With 1H-imidazole In N,N-dimethyl-formamide for 4.5h; | 86% |
| With 1H-imidazole In tetrahydrofuran | 60% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide; dmap In dichloromethane at 20℃; for 2h; | 89% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide; dmap In dichloromethane at 20℃; for 2h; | 89% |
-
-
20826-04-4
5-bromo-3-pyridinecarboxylic acid

-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
888029-94-5
C27H22BrNO9

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide; dmap In dichloromethane at 20℃; for 2h; | 87% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide; dmap In dichloromethane at 20℃; for 2h; | 87% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 87% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
501-53-1
benzyl chloroformate

-
-
23412-22-8
4'-benzyloxycarbonyl-4'-demethylpodophyllotoxin

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 85.65% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 24.25h; Cooling with ice; | 85% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| Stage #1: 4'-demethylepipodophyllotoxin With boron trifluoride diethyl etherate In dichloromethane at 0℃; for 0.166667h; Inert atmosphere; Stage #2: 3,4,6-tri-O-acetyl-2-azido-2-deoxy-β-D-glucopyranose In dichloromethane at -20℃; for 2h; Inert atmosphere; Molecular sieve; | 85% |
| Stage #1: 4'-demethylepipodophyllotoxin With boron trifluoride diethyl etherate In dichloromethane at 0℃; Inert atmosphere; Stage #2: 3,4,6-tri-O-acetyl-2-azido-2-deoxy-β-D-glucopyranose In dichloromethane at -5℃; for 4h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide; dmap In dichloromethane at 20℃; for 2h; | 83% |
-
-
5326-23-8
6-Chloro-3-pyridinecarboxylic acid

-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
888029-93-4
C27H22ClNO9

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide; dmap In dichloromethane at 20℃; for 2h; | 83% |
-
-
6559-91-7
4'-demethylepipodophyllotoxin

-
-
138261-31-1
2'-chloro-4′-demethylepipodophyllotoxin

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In N,N-dimethyl-formamide at 0 - 20℃; for 18h; | 82% |
| With N-chloro-succinimide In N,N-dimethyl-formamide for 3.5h; Ambient temperature; | 75% |
| With N-chloro-succinimide In chloroform at 20℃; for 4h; regioselective reaction; | |
| With N-chloro-succinimide In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 82% |
4'-Demethylepipodophyllotoxin Specification
The CAS registry number of 4'-Demethylepipodophyllotoxin is 6559-91-7. The IUPAC name is (5S,5aR,8aR,9R)-5-hydroxy-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one. It belongs to the classes of Miscellaneous Natural Products; Plant Oils, Toxins, Phenolic Acids & Derivatives; Inhibitors. In addition, its molecular formula is C21H20O8 and the molecular weight is 400.38. Besides, it is off-white solid. And it can be used as a potent inhibitor of microtubule assembly.
Physical properties about this chemical are: (1)ACD/LogP: 0.71; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.71; (4)ACD/LogD (pH 7.4): 0.71; (5)ACD/BCF (pH 5.5): 2.05; (6)ACD/BCF (pH 7.4): 2.04; (7)ACD/KOC (pH 5.5): 58.17; (8)ACD/KOC (pH 7.4): 58; (9)H bond acceptors: 8; (10)H bond donors: 2; (11)Freely Rotating Bonds: 5; (12)Polar Surface Area: 81.68 Å2; (13)Index of Refraction: 1.637; (14)Molar Refractivity: 99.48 cm3; (15)Molar Volume: 276.8 cm3; (16)Polarizability: 39.43×10-24cm3; (17)Surface Tension: 60.3 dyne/cm; (18)Density: 1.446 g/cm3; (19)Flash Point: 224.4 °C; (20)Enthalpy of Vaporization: 97.48 kJ/mol; (21)Boiling Point: 626.5 °C at 760 mmHg; (22)Vapour Pressure: 1.46E-16 mmHg at 25 °C.
Preparation of 4'-Demethylepipodophyllotoxin: it can be prepared by 1-Brom-1-desoxy-4'-demethyl-epipodophyllotoxin. This reaction will need reagents BaCO3 and H2O and solvent acetone. The reaction time is 4 hours at reaction temperature of 40 °C. The yield is about 71%.
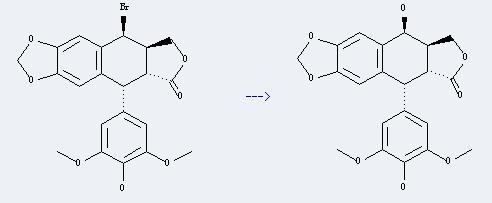
Uses of 4'-Demethylepipodophyllotoxin: it can be used to get 2'-chloro-4'-O-demethylepipodophyllotoxin. This reaction will need reagent N-chlorosuccinimide and solvent dimethylformamide. The reaction time is 3.5 hours at ambient temperature. The yield is about 75%.

People can use the following data to convert to the molecule structure.
(1)SMILES: O=C4OC[C@@H]5[C@H](O)c2cc1OCOc1cc2[C@@H](c3cc(OC)c(O)c(OC)c3)[C@@H]45
(2)InChI: InChI=1/C21H20O8/c1-25-15-3-9(4-16(26-2)20(15)23)17-10-5-13-14(29-8-28-13)6-11(10)19(22)12-7-27-21(24)18(12)17/h3-6,12,17-19,22-23H,7-8H2,1-2H3/t12-,17+,18-,19+/m0/s1
(3)InChIKey: YVCVYCSAAZQOJI-JHQYFNNDBA
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View