-
Name
4'-Hydroxypropiophenone
- EINECS 200-743-2
- CAS No. 70-70-2
- Article Data72
- CAS DataBase
- Density 1.104 g/cm3
-
Solubility
0.34 g/l (15 °C) in water
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms POP;Paroxon;Paroxypropion;Propiophenone,4'-hydroxy- (8CI);Propiophenone, p-hydroxy- (3CI);1-(4-Hydroxyphenyl)-1-propanone;1-(p-Hydroxyphenyl)-1-propanone;4-Propanoylphenol;4-Propionylphenol;Paroxypropione;B 360;Bio-fren;Ethyl 4-hydroxyphenyl ketone;Ethyl p-hydroxyphenyl ketone;Frenantol;Frenohypon;Frenon;Frenormon;H 365;Hypophenon;Hypostat;Ibiopopp;Mepal;NSC 2834;NSC 33949;
- PSA 37.30000
- LogP 1.98490
Synthetic route

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 0 - 20℃; for 1h; Friedel-Crafts acylation; regiospecific reaction; | 99% |
| With aluminium trichloride In dichloromethane Friedel-Crafts acylation; | 53% |
| man verseift das Propionat mit alkoh. Kalilauge; |

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With cerium (IV) sulfate tetrahydrate In methanol at 130℃; for 0.333333h; Temperature; Microwave irradiation; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In isopropyl alcohol at 60℃; under 3102.9 Torr; 20-24 h; | 92% |
| With trifluoroacetic acid |
-
-
69405-03-4
4-{[(tert-butyl)(diphenyl)silyl]oxy}-3-methoxybenzaldehyde

-
-
134136-83-7
1-(4-{[tert-butyl(dimethyl)silyl]oxy}phenyl)propan-1-one

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With cerium (IV) sulfate tetrahydrate In methanol at 130℃; for 0.333333h; Microwave irradiation; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Methoxypropiophenone With 1-n-butyl-3-methylimidazolim bromide at 220℃; under 9000.9 Torr; for 0.666667h; Inert atmosphere; Sealed tube; Microwave irradiation; Stage #2: With hydrogenchloride; water pH=1; | 90% |
| With hydrogen bromide; acetic acid In water at 100℃; for 18h; Heating / reflux; | 70% |
| With Pyridine hydrobromide at 240℃; for 0.25h; | 60% |
-
-
192067-89-3
1-(4-(prop-2-yn-1-yloxy)phenyl)propan-1-one

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With benzyltriethylammonium tetrathiomolybdate In acetonitrile at 28℃; for 19h; | 87% |
| With Pd-catalyst In water; N,N-dimethyl-formamide |
-
-
22805-42-1
4-hydroxy-α-ethylbenzylalcohol

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With C6H4MoNO7(1-)*C19H42N(1+); oxygen In water at 100℃; for 20h; Green chemistry; chemoselective reaction; | 81% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 0 - 20℃; for 4.5h; Inert atmosphere; | 80% |
| With trifluorormethanesulfonic acid at 0 - 20℃; for 4.5h; | 80% |
| With aluminium trichloride In nitrobenzene at 30℃; for 24h; Fries rearrangement; | 60% |

| Conditions | Yield |
|---|---|
| Stage #1: propionic acid With trifluoromethylsulfonic anhydride at 20 - 60℃; Stage #2: phenol at 60℃; for 0.166667h; regiospecific reaction; | 80% |
| With zinc(II) chloride | |
| With boron trifluoride | |
| With PPA |
-
-
10342-83-3
4'-Bromopropiophenone

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With nickel(II) bromide trihydrate; water; 1-(2,2-diphenyl-2λ4,3λ4-[1,3,2]diazaborolo[4,5,1-ij]quinolin-1(2H)-yl)-3-phenylpropan-1-one; N-ethyl-N,N-diisopropylamine; 4,4'-di-tert-butyl-2,2'-bipyridine In dimethyl sulfoxide at 25℃; for 22h; Irradiation; | 80% |
-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
99-93-4
4-Hydroxyacetophenone

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer In water at 110℃; for 3h; Inert atmosphere; Sealed tube; | 73% |
-
-
637-27-4
phenyl propionate

-
A
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With aluminium trichloride In chlorobenzene at 106℃; under 760 Torr; for 0.05h; Irradiation; microwave oven; | A 28% B 62% |
| With carbon disulfide; aluminium trichloride Erhitzen des Reaktionsgemisches auf 190grad; | |
| With carbon disulfide; aluminium trichloride bei Siedetemperatur und anschliessend Abdestillieren des Schwefelkohlenstoffs und Erhitzen auf 140-150grad; | |
| With aluminium trichloride; n-heptane Erhitzen des Reaktionsgemisches auf 190grad; |
-
-
637-27-4
phenyl propionate

-
A
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
B
-
70-70-2
4-hydroxypropiophenone

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With β‐cyclodextrin In water at 25℃; for 1h; Product distribution; Quantum yield; Irradiation; various times, effect of oxygen, without cyclodextrin; | A 16.49% B 9.16% C n/a |

-
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
A
-
637-27-4
phenyl propionate

-
B
-
74316-25-9
2-chloro-1-(2-hydroxyphenyl)-1-propanone

-
C
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| iron(III) chloride at 170℃; for 24h; Mechanism; Product distribution; | A 8.7% B 5.9% C 0.7% |

-
-
60-29-7
diethyl ether

-
-
142-96-1
dibutyl ether

-
-
79119-31-6
N, N-diethyl-4-hydroxybenzamide

-
-
925-90-6
ethylmagnesium bromide

-
-
70-70-2
4-hydroxypropiophenone

-
-
79119-31-6
N, N-diethyl-4-hydroxybenzamide

-
-
925-90-6
ethylmagnesium bromide

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With diethyl ether; dibutyl ether Erhitzen des vom Diaethylaether befreiten Reaktionsgemisches auf Siedetemperatur und anschliessende Hydrolyse; |
-
-
80-46-6
4-t-amylphenol

-
-
802294-64-0
propionic acid

-
A
-
859059-71-5
1-(2-hydroxy-5-tert-pentyl-phenyl)-propan-1-one

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With boron trifluoride |

-
-
802294-64-0
propionic acid

-
-
108-95-2
phenol

-
A
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With boron trifluoride Erwaermen des Reaktionsgemisches auf 75grad; | |
| With boron trifluoride und Erwaermen des Reaktionsgemisches auf 75grad; |


| Conditions | Yield |
|---|---|
| With N-butylamine In benzene for 0.4h; Ambient temperature; Yield given; |
-
-
96187-97-2
4-propionylphenyl benzoate

-
-
109-73-9
N-butylamine

-
A
-
2782-40-3
N-butylbenzamide

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| In benzene for 0.4h; Ambient temperature; Yields of byproduct given; |

-
-
134136-83-7
1-(4-{[tert-butyl(dimethyl)silyl]oxy}phenyl)propan-1-one

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate; [2.2.2]cryptande In acetonitrile at 55℃; for 2h; Yield given; | |
| With tetrabutyl ammonium fluoride In tetrahydrofuran |
-
-
7637-07-2
boron trifluoride

-
-
802294-64-0
propionic acid

-
-
108-95-2
phenol

-
A
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| at 73 - 75℃; | |
| at 75℃; |

-
-
53946-87-5
2-bromo-(4'-hydroxyphenyl)-propan-1-one

-
-
64-19-7
acetic acid

-
-
70-70-2
4-hydroxypropiophenone

-
-
75-15-0
carbon disulfide

-
-
7446-70-0
aluminium trichloride

-
-
637-27-4
phenyl propionate

-
A
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| Erhitzen des vom Loesungsmittel befreiten Reaktionsgemisches auf 135-140grad; |

-
-
7446-70-0
aluminium trichloride

-
-
142-82-5
n-heptane

-
-
637-27-4
phenyl propionate

-
A
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| at 80 - 90℃; Product distribution; |

-
-
7446-70-0
aluminium trichloride

-
-
637-27-4
phenyl propionate

-
-
79-34-5
1,1,2,2-tetrachloroethane

-
A
-
610-99-1
1-(2-Hydroxy-phenyl)-propan-1-on

-
B
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| at 95℃; Product distribution; |

-
-
56-23-5
tetrachloromethane

-
-
84234-15-1
bis-[1-(4-hydroxy-phenyl)-propylidene]-hydrazine

-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| in siedendem Xylol, nach laengerer Dauer; |

-
-
13154-24-0
triisopropylsilyl chloride

-
-
70-70-2
4-hydroxypropiophenone

-
-
134136-88-2
1-(4-{[tris(1-methylethyl)silyl]oxy}phenyl)propan-1-one

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0 - 20℃; for 14h; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide for 3h; | 99% |
-
-
3282-30-2
pivaloyl chloride

-
-
70-70-2
4-hydroxypropiophenone

-
-
120703-45-9
p-(trimethylacetoxy)propiophenone

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 2h; | 100% |
| Stage #1: 4-hydroxypropiophenone With sodium hydride In tetrahydrofuran; mineral oil Stage #2: pivaloyl chloride In tetrahydrofuran; mineral oil for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Heating / reflux; | 100% |
| With potassium carbonate In acetone Heating / reflux; | 100% |
| With potassium carbonate In acetone Heating / reflux; | 100% |

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol at 80℃; for 4h; | 99% |
| With sodium acetate at 80℃; for 2h; |
-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
70-70-2
4-hydroxypropiophenone

| Conditions | Yield |
|---|---|
| With indium iodide In tetrahydrofuran at 40℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1,2-dimethoxyethane at 145℃; for 0.416667h; Microwave irradiation; Inert atmosphere; Sealed vessel; | 98% |
| With potassium carbonate at 150 - 160℃; for 4h; | 81% |
-
-
107-21-1
ethylene glycol

-
-
70-70-2
4-hydroxypropiophenone

-
-
89723-36-4
2-ethyl-2-(4-hydroxyphenyl)-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With Triisopropoxymethan; cerium triflate In hexane at 25℃; for 2.5h; | 98% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 10 - 20℃; for 2h; Temperature; Concentration; | 97.3% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; for 12h; | 97% |
| at 75 - 90℃; for 6h; Substitution; | 89.4% |
| With sodium hydroxide |
-
-
105-36-2
ethyl bromoacetate

-
-
70-70-2
4-hydroxypropiophenone

-
-
51828-70-7
ethyl 2-(4-propionylphenoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 4h; Heating / reflux; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 3h; | 94% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 3h; | 94% |
| With potassium carbonate In toluene for 48h; Reflux; |
-
-
824-94-2
p-methoxybenzyl chloride

-
-
70-70-2
4-hydroxypropiophenone

-
-
935859-44-2
1-{4-[(4-methoxybenzyl)oxy]phenyl}propan-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In butanone at 20 - 60℃; for 12h; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; for 12h; Inert atmosphere; |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
70-70-2
4-hydroxypropiophenone

-
-
51828-70-7
ethyl 2-(4-propionylphenoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 18h; | 95% |
-
-
70-70-2
4-hydroxypropiophenone

-
-
65440-82-6
O-(4-bromophenyl)hydroxylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol at 20℃; for 2h; | 94% |
-
-
70-70-2
4-hydroxypropiophenone

-
-
504-63-2
trimethyleneglycol

-
-
1171247-83-8
2-ethyl-2-(4-hydroxyphenyl)-1,3-dioxane

| Conditions | Yield |
|---|---|
| With Triisopropoxymethan; cerium triflate In hexane at 25℃; for 6h; | 94% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 93% |
-
-
124-63-0
methanesulfonyl chloride

-
-
70-70-2
4-hydroxypropiophenone

-
-
929431-57-2
4-propionyl-phenyl methanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 5℃; for 1.5h; | 93% |
-
-
869-24-9
2-chloro-N,N-diethylethylamine hydrochloride

-
-
70-70-2
4-hydroxypropiophenone

-
-
92726-49-3
1-(4-(2-(diethylamino)ethoxy)phenyl)propan-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Heating; | 92% |
| With potassium carbonate In butanone |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 92% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 91.5% |
| With potassium carbonate In acetone Heating; | |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Heating; | 91% |
| With potassium carbonate In acetone at 60℃; for 6h; | 72% |
-
-
70-70-2
4-hydroxypropiophenone

-
-
53946-87-5
2-bromo-(4'-hydroxyphenyl)-propan-1-one

| Conditions | Yield |
|---|---|
| With pyridinium hydrobromide perbromide; acetic acid at 20℃; for 3.08333h; Product distribution / selectivity; | 90% |
| With pyridinium hydrobromide perbromide; acetic acid at 20℃; for 3h; | 66% |
| With bromine In chloroform; ethyl acetate | 61.3% |

| Conditions | Yield |
|---|---|
| palladium on activated charcoal In ethanol at 34℃; under 37503 Torr; for 0.833333h; | 90% |
| With palladium on activated charcoal; ethanol Hydrogenation; | |
| With methanol; copper oxide-chromium oxide at 115 - 125℃; under 161812 - 176522 Torr; Hydrogenation; |
-
-
25866-35-7
4-chloro-2,3-(hexamethylene)quinoline

-
-
70-70-2
4-hydroxypropiophenone

-
-
108154-92-3
1-[4-(6,7,8,9,10,11-Hexahydro-cycloocta[b]quinolin-12-yloxy)-phenyl]-propan-1-one

| Conditions | Yield |
|---|---|
| at 140℃; for 24h; | 90% |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
70-70-2
4-hydroxypropiophenone

-
-
87241-55-2
4-propionylphenol trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; dmap In dichloromethane for 2h; Ambient temperature; | 90% |
| With dmap In dichloromethane at -20 - 20℃; Inert atmosphere; | 90% |
| With pyridine In dichloromethane at 0 - 20℃; | 88% |
| With pyridine In dichloromethane at 0 - 20℃; Inert atmosphere; | 88% |
| With 2,6-dimethylpyridine; dmap In dichloromethane at 20℃; for 2h; | 14 g |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 85℃; | 90% |
4'-Hydroxypropiophenone Chemical Properties
Molecule structure of Paroxypropione (CAS NO.70-70-2):
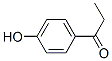
IUPAC Name: 1-(4-Hydroxyphenyl)propan-1-one
Molecular Formula: C9H10O2
Molecular Weight: 150.174500 g/mol
Melting Point: 36-38 °C(lit.)
Density: 1.104 g/cm3
Melting Point: 36-38 °C(lit.)
Boiling Point: 152-154 °C at 26 mm Hg(lit.)
Flash Point: >230 °F
Index of Refraction: 1.542
Molar Refractivity: 42.79 cm3
Molar Volume: 135.9 cm3
Surface Tension: 42.6 dyne/cm
Methanol Solubility: 0.1 g/mL, clear
Water Solubility: 0.34 g/l (15 °C)
XLogP3: 2
H-Bond Donor: 1
H-Bond Acceptor: 2
Rotatable Bond Count: 2
Tautomer Count: 7
Exact Mass: 150.06808
MonoIsotopic Mass: 150.06808
Topological Polar Surface Area: 37.3
Heavy Atom Count: 11
Canonical SMILES: CCC(=O)C1=CC=C(C=C1)O
InChI: InChI=1S/C9H10O2/c1-2-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3
InChIKey: RARSHUDCJQSEFJ-UHFFFAOYSA-N
EINECS: 202-815-9
Product Categories: Acetophenone Series; Aromatic Propiophenones (substituted); C9; Carbonyl Compounds; Ketones
4'-Hydroxypropiophenone Uses
Paroxypropione (CAS NO.70-70-2) is used as an intermediates of liquid crystals.
4'-Hydroxypropiophenone Toxicity Data With Reference
| 1. | ims-rbt TDLo:1600 mg/kg (female 15-30D post):TER | AOGNAX Archivio di Ostetricia e Ginecologia. 66 (1961),286. | ||
| 2. | orl-mus LD50:3 mg/kg | 85JDAH Organophosphorus Pesticides: Organic and Biological Chemistry Eto, M.,Cleveland, OH.: CRC Press, Inc.,1974,197. | ||
| 3. | ipr-mus LD50:200 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . | ||
| 4. | scu-mus LD50:1130 µg/kg | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 124 (1960),212. | ||
| 5. | par-frg LD50:91 mg/kg | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 124 (1960),212. |
4'-Hydroxypropiophenone Consensus Reports
Reported in EPA TSCA Inventory.
4'-Hydroxypropiophenone Safety Profile
Hazard Codes:  Xn,
Xn,  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 37-24/25-36-26
S37:Wear suitable gloves.
S24/25:Avoid contact with skin and eyes.
S36:Wear suitable protective clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 3
RTECS: UH1925000
HS Code: 29145000
Hazardous Substances Data: 70-70-2(Hazardous Substances Data)
Poison by intraperitoneal, subcutaneous, and parenteral routes. An experimental teratogen. Other experimental reproductive effects. A flammable liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also KETONES.
4'-Hydroxypropiophenone Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
4'-Hydroxypropiophenone Specification
Paroxypropione (CAS NO.70-70-2) is also named as 1-(4-Hydroxyphenyl)-1-propanone ; 1-(p-Hydroxyphenyl)-1-propanone ; 4-08-00-00441 (Beilstein Handbook Reference) ; 4-Hydroxypropiophenone ; 4-Propionylphenol ; AI3-03719 ; B 360 ; Ethyl p-hydroxyphenyl ketone ; Frenantol ; Frenohypon ; Frenon ; Frenormon ; H 365 ; Paroxipropiona ; Paroxipropiona [INN-Spanish] ; Paroxon ; Possipione ; Profenone ; Proxiphenon ; Sterofenon ; UNII-X9952001TG ; USAF EK-3302 ; p-Hydroxyphenyl-1-propanone ; p-Hydroxypropiophenone ; p-Oxypropiophenone ; p-Propionylphenol ; p-Propiophenol . Paroxypropione (CAS NO.70-70-2) is white powder. It is slightly soluble in water, soluble in boiling water, with alcohol, ether immiscibility.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View