-
Name
4-Amino-3-nitrophenol
- EINECS 210-236-8
- CAS No. 610-81-1
- Article Data14
- CAS DataBase
- Density 1.511 g/cm3
- Solubility Soluble in water
- Melting Point 150-154 °C(lit.)
- Formula C6H6N2O3
- Boiling Point 374.4 °C at 760 mmHg
- Molecular Weight 154.125
- Flash Point 180.3 °C
- Transport Information UN 2811
- Appearance dark red powder
- Safety 26-36-37/39
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 2-Amino-5-hydroxynitrobenzene;2-Nitro-4-hydroxyaniline;Phenol, 4-amino-3-nitro-;
- PSA 92.07000
- LogP 1.98700
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine; silver nitrate; triphenylphosphine In acetonitrile at 20℃; for 0.0833333h; | A 40% B 50% |

-
-
610-81-1
4-hydroxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| Stage #1: N-(4-hydroxy-2-nitrophenyl)-acetamide With thionyl chloride In methanol at 70℃; for 0.5h; Stage #2: With potassium carbonate In methanol at 20℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide | |
| With hydrogen bromide | |
| Multi-step reaction with 2 steps 1: bromine; acetic acid 2: concentrated aqueous hydrochloric acid View Scheme |

-
-
610-81-1
4-hydroxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
-
610-81-1
4-hydroxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
7664-93-9
sulfuric acid

-
-
861560-26-1
2-nitro-toluene-4-sulfonic acid-(4-ethoxy-2-nitro-anilide)

-
-
610-81-1
4-hydroxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| at 80 - 90℃; |
-
-
7664-93-9
sulfuric acid

-
A
-
97-06-3
4-methyl-3-nitro-benzenesulfonic acid

-
B
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
450365-10-3
1-acetoxy-4-(toluene-4-sulfonylamino)-benzene

-
-
108-24-7
acetic anhydride

-
A
-
610-81-1
4-hydroxy-2-nitroaniline

-
B
-
32654-60-7
4-amino-3,5-dinitrophenol


-
-
80824-77-7
4'-(benzyloxy)benzanilide

-
-
610-81-1
4-hydroxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: nitric acid / 0 - 10 °C 2: sulfuric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: natrium carbonate 2: concentrated sulfuric acid; nitric acid / <17 3: concentrated sulfuric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid; nitric acid / <17 2: concentrated sulfuric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: nitric acid 2: NaOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) (diazotization), (ii) aq. NaN3 2: aq. H2SO4 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: nitric acid; boron trifluoride diethyl etherate; bis-[(trifluoroacetoxy)iodo]benzene; acetic acid / water / 2.5 h / 60 °C 2.1: thionyl chloride / methanol / 0.5 h / 70 °C 2.2: 0.5 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In ethanol for 3.5h; | 100% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; for 3.5h; | 100% |
| With hydrazine hydrate; nickel In ethanol at 20℃; for 0.5h; | 96% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
215656-99-8
4-{[tert-butyl(dimethyl)silyl]oxy}-2-nitroaniline

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 20℃; Cooling with ice; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
639084-14-3
3-tert-butyldimethylsiloxy-2-amino-1-nitrobenzene

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 18h; | 100% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl-diazodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 100% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
215656-99-8
4-{[tert-butyl(dimethyl)silyl]oxy}-2-nitroaniline

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
13154-24-0
triisopropylsilyl chloride

-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
1262887-34-2
2-nitro-4-((triisopropylsilyl)oxy)aniline

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; | 99% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
104-12-1
p-chlorphenylisocyanate

-
-
862014-82-2
1-(4-chlorophenyl)-3-(4-hydroxy-2-nitro-phenyl)urea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; dichloromethane at 20℃; for 20h; | 98% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
1414615-74-9
2-(4-amino-3-nitrophenoxy)acetonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; chemoselective reaction; | 98% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
105-36-2
ethyl bromoacetate

-
-
59820-64-3
ethyl 2-(4-amino-3-nitrophenoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 25℃; for 8h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide for 2.5h; | 94% |
| With potassium carbonate In acetone at 20℃; | 75.2% |
| With potassium carbonate In acetone at 20℃; Inert atmosphere; | 75.2% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
3386-35-4
1-octyl p-toluenesulfonate

-
-
1018902-72-1
2-nitro-4-octyloxyaniline

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Reflux; | 98% |
-
-
420-04-2
CYANAMID

-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
157284-07-6
3-amino-7-hydroxybenzo[e][1,2,4]triazine 1-oxide

| Conditions | Yield |
|---|---|
| Stage #1: CYANAMID; 4-hydroxy-2-nitroaniline at 100℃; for 0.166667h; Stage #2: With hydrogenchloride In water at 20 - 100℃; for 1.5h; Stage #3: With sodium hydroxide In water at 20 - 100℃; for 1h; | 97% |
| Stage #1: CYANAMID; 4-hydroxy-2-nitroaniline In water; acetic acid for 48h; Heating / reflux; Stage #2: With sodium hydroxide In water; acetic acid Heating / reflux; | 51.7% |
-
-
288-32-4
1H-imidazole

-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
215656-99-8
4-{[tert-butyl(dimethyl)silyl]oxy}-2-nitroaniline

| Conditions | Yield |
|---|---|
| In ethyl acetate; N,N-dimethyl-formamide | 97% |
| In ethyl acetate; N,N-dimethyl-formamide | 97% |
| In ethyl acetate; N,N-dimethyl-formamide | 97% |
| In N,N-dimethyl-formamide | 26.5 g (76%) |

-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
157284-07-6
3-amino-7-hydroxybenzo[e][1,2,4]triazine 1-oxide

| Conditions | Yield |
|---|---|
| 97% | |
| 93% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
1414615-68-1
C13H10BrFN2O3

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; chemoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With tin(ll) chloride at 130℃; for 0.0833333h; microwave; | 95% |
| With iron; ammonium chloride In isopropyl alcohol at 80℃; for 1h; | 87% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
210832-86-3
6-bromoacetyl-2-dimethylaminonaphthalene

-
-
1552301-13-9
C20H19N3O4

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 4h; Inert atmosphere; | 95% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
201811-20-3
tert-butyl N-(4-hydroxy-2-nitrophenyl)carbamate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 25℃; for 2h; | 95% |
-
-
31643-49-9
4-Nitrophthalonitrile

-
-
610-81-1
4-hydroxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 26h; | 94% |
-
-
14400-67-0
2,5-dimethyl-2,3-dihydrofuran-3-one

-
-
610-81-1
4-hydroxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxy-2-nitroaniline With hydrogenchloride; sodium nitrite at 0℃; for 1h; Stage #2: 2,5-dimethyl-2,3-dihydrofuran-3-one In water at 20℃; for 1h; | 93% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In ethyl acetate | 92% |
| With diisopropyl (E)-azodicarboxylate; triphenylphosphine In tetrahydrofuran | |
| With diisopropyl (E)-azodicarboxylate; triphenylphosphine In tetrahydrofuran |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
1261238-07-6
tert-butyl 5-(4-amino-3-nitrophenoxy)pentylcarbamate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxy-2-nitroaniline With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; Inert atmosphere; Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil at 20℃; for 24h; Inert atmosphere; | 90% |
| Stage #1: 4-hydroxy-2-nitroaniline With sodium hydride In N,N-dimethyl-formamide; mineral oil for 0.5h; Cooling with ice; Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil at 20℃; for 12h; | 89% |
| With potassium carbonate In acetone |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
74388-24-2
1-amino-4-(4-chloro-phenylmethoxy)-2-nitro-benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; chemoselective reaction; | 90% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
254454-54-1
tert-butyl-3-iodoazetidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 90℃; for 16h; Inert atmosphere; | 90% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
6315-52-2
1,2-bis-tosyloxyethane

-
-
67499-48-3
4,4'-(ethane-1,2-diylbis(oxy))bis(2-nitroaniline)

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 6h; | 89.2% |
| With potassium carbonate In acetonitrile at 80℃; for 24h; | 69% |
-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
113305-56-9
4-iodo-3-nitrophenol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium iodide; sodium nitrite | 89% |
| Stage #1: 4-hydroxy-2-nitroaniline With hydrogenchloride; sodium nitrite In water at 0℃; for 0.5h; Stage #2: With potassium iodide In water at 0℃; for 3.5h; Darkness; | 89% |
| Stage #1: 4-hydroxy-2-nitroaniline With hydrogenchloride; sodium nitrite at 0℃; for 1h; Stage #2: With potassium iodide In water at 20℃; Sandmayer reaction; | 83% |
-
-
499-06-9
3,5-dimethylbenzoic acid

-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
197223-18-0
4-amino-3-nitrophenyl 3,5-dimethylbenzoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane for 15h; Esterification; Heating; | 89% |
-
-
6972-71-0
4,5-dimethyl-2-nitrobenzenamine

-
-
610-81-1
4-hydroxy-2-nitroaniline

-
-
251649-40-8
1-(4-hydroxy-2-nitrophenyl)-1H-pyrrole

| Conditions | Yield |
|---|---|
| With acetic acid for 1h; Clauson-Kaas reaction; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With sodium dithionite In ethanol at 80℃; for 12h; | 89% |

| Conditions | Yield |
|---|---|
| With tin(ll) chloride at 130℃; for 0.0833333h; microwave; | 89% |
4-Amino-3-nitrophenol Chemical Properties
Molecular structure of 4-Amino-3-nitrophenol (CAS NO.610-81-1) is:
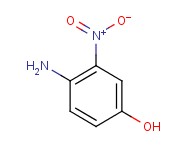
Product Name: 4-Amino-3-nitrophenol
CAS Registry Number: 610-81-1
IUPAC Name: 4-amino-3-nitrophenol
Molecular Weight: 154.12344 [g/mol]
Molecular Formula: C6H6N2O3
XLogP3: 0.9
H-Bond Donor: 2
H-Bond Acceptor: 4
EINECS: 210-236-8
Melting Point: 150-154 °C(lit.)
Water Solubility: soluble
Surface Tension: 78.5 dyne/cm
Density: 1.511 g/cm3
Flash Point: 180.3 °C
Enthalpy of Vaporization: 64.62 kJ/mol
Boiling Point: 374.4 °C at 760 mmHg
Vapour Pressure: 3.87E-06 mmHg at 25°C
Product Categories: Aromatic Phenols;Phenol&Thiophenol&Mercaptan;Phenoles and thiophenoles
4-Amino-3-nitrophenol Safety Profile
Safty information about 4-Amino-3-nitrophenol (CAS NO.610-81-1) is:
Hazard Codes:  Xi,Xn
Xi,Xn
Risk Statements: 36/37/38-20/21/22
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
RIDADR: 2811
WGK Germany: 3
PackingGroup: III
HS Code: 29222900
4-Amino-3-nitrophenol Specification
4-Amino-3-nitrophenol , its cas register number is 610-81-1. It also can be called 4-Amino-3-nitrophenol ; Phenol, 4-amino-3-nitro- .It is a dark red powder.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View