-
Name
4-Bromo-1H-imidazole
- EINECS
- CAS No. 2302-25-2
- Article Data18
- CAS DataBase
- Density 1.904 g/cm3
- Solubility Slightly soluble in water.
- Melting Point 131-135 °C(lit.)
- Formula C3H3BrN2
- Boiling Point 324.7 °C at 760 mmHg
- Molecular Weight 146.974
- Flash Point 150.2 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance Colorless to beige crystalline flakes or powder
- Safety 26-36/37/39-45
- Risk Codes 25-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 1H-Imidazole,4-bromo- (9CI);Imidazole, 4(or 5)-bromo- (6CI,7CI);Imidazole, 4-bromo- (8CI);4(or 5)-Bromoimidazole;4-Bromoimidazole;NSC 227280;NSC54254;
- PSA 28.68000
- LogP 1.17220
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium sulfite In water at 110℃; for 6h; | 89% |
| With sodium sulfite In water at 110℃; for 6h; | 85% |
| With water; sodium sulfite | |
| With sodium sulfite Heating; |
-
-
64591-03-3
2,4-dibromo-1H-imidazole

-
-
2302-25-2
4-bromo-1 H-imidazole

| Conditions | Yield |
|---|---|
| With sodium sulfite In water at 100℃; for 1h; Sealed tube; Microwave irradiation; Green chemistry; | 71% |
| With water; sodium sulfite |
-
-
288-32-4
1H-imidazole

-
A
-
2302-30-9
4,5-dibromo-1H-imidazole

-
B
-
2034-22-2
2,4,5-tribromo-1H-imidazole

-
C
-
2302-25-2
4-bromo-1 H-imidazole

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 20℃; for 48h; Yields of byproduct given; | A n/a B n/a C 41% |
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 20℃; for 48h; | A 6% B 3% C 32% |

-
-
288-32-4
1H-imidazole

-
-
77-48-5
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione

-
A
-
2302-25-2
4-bromo-1 H-imidazole

-
B
-
77-71-4
5,5-dimethyl-imidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 0℃; for 0.0833333h; | A 21% B 33% |


| Conditions | Yield |
|---|---|
| With potassium (aquo)(2‑chlorobenzoato)oxodiperoxo–tungstate(VI) dihydrate; potassium bromide In water; acetonitrile at 20℃; | A 27% B 16% |

| Conditions | Yield |
|---|---|
| With water; sodium sulfite | |
| With sodium sulfite In water; isopropyl alcohol Reflux; Large scale; | 3.6 kg |
-
-
39870-05-8
4-acetyl pyrimidine

-
A
-
2302-25-2
4-bromo-1 H-imidazole

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 24.9℃; Equilibrium constant; |

-
-
861362-49-4
5-bromo-1(3)H-imidazole-4-carboxylic acid-(4-bromo-anilide)

-
-
2302-25-2
4-bromo-1 H-imidazole

| Conditions | Yield |
|---|---|
| at 150℃; |

-
-
2302-25-2
4-bromo-1 H-imidazole

| Conditions | Yield |
|---|---|
| With hydrogen bromide at 150℃; im Rohr; |
-
-
64591-03-3
2,4-dibromo-1H-imidazole

-
-
2302-25-2
4-bromo-1 H-imidazole
-
-
7647-01-0
hydrogenchloride

-
-
116081-72-2
5-bromo-1(3)H-imidazole-4-sulfonic acid

-
A
-
2302-25-2
4-bromo-1 H-imidazole

-
B
-
7664-93-9
sulfuric acid

| Conditions | Yield |
|---|---|
| at 170℃; |

-
-
2034-22-2
2,4,5-tribromo-1H-imidazole

-
-
7732-18-5
water

-
A
-
288-32-4
1H-imidazole

-
B
-
2302-30-9
4,5-dibromo-1H-imidazole

-
C
-
2302-25-2
4-bromo-1 H-imidazole


| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In water at 28.44℃; Kinetics; Thermodynamic data; Temperature; | |
| With bromine In water at 28.44℃; Kinetics; |

| Conditions | Yield |
|---|---|
| for 0.5h; | 99% |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1338257-80-9
4-bromo-imidazole-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 2h; | 98% |
| With dmap In tetrahydrofuran at 25℃; for 1.5h; | 95% |
| With dmap In dichloromethane at 20℃; for 12h; | 93% |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
898830-80-3
4-bromo-1-(tetramethylsilyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| for 6h; Heating; | 95% |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
192182-54-0
3,5-dimethoxyphenylboronic acid

-
-
312583-37-2
4(5)-(3,5-dimethoxyphenyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; N-benzyl-N,N,N-triethylammonium chloride; cesium fluoride In water; toluene for 96h; Suzuki-Miyaura Coupling; Inert atmosphere; Reflux; | 95% |
-
-
2302-25-2
4-bromo-1 H-imidazole


| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 5h; | 93% |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
459-64-3
4-methoxybenzenediazonium tetrafluoroborate

-
-
130749-87-0
4-bromo-2-(4-methoxyphenylazo)imidazole

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 0 - 5℃; for 1h; | 92% |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
139911-27-6
4-fluoro-3-methylphenyl boronic acid

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In 1,2-dimethoxyethane; ethanol; water at 150℃; for 0.833333h; Inert atmosphere; Microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 24h; Microwave irradiation; | 92% |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
76513-69-4
(2-trimethylethylsilylethoxy)methyl chloride

-
-
211615-79-1
4-bromo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-1 H-imidazole With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In tetrahydrofuran; mineral oil at 0 - 20℃; for 18h; Inert atmosphere; | 91.3% |
| Stage #1: 4-bromo-1 H-imidazole With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.5h; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In tetrahydrofuran; mineral oil at 20℃; for 2h; | 1.36 g |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
35512-31-3
4-(4-methoxyphenyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; cesium fluoride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In water; toluene at 110℃; for 66h; Suzuki-Miyaura reaction; | 91% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; N-benzyl-N,N,N-triethylammonium chloride; cesium fluoride In water; toluene for 96h; Suzuki-Miyaura Coupling; Inert atmosphere; Reflux; | 75% |
-
-
2302-25-2
4-bromo-1 H-imidazole

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; water-d2 In water-d2 at 100℃; for 1h; | 91% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 90℃; | 90.6% |
-
-
2302-25-2
4-bromo-1 H-imidazole

-
-
32316-92-0
naphthalene-2-boronic acid

-
-
54532-16-0
4(5)-(2-naphthyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; cesium fluoride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In water; toluene at 110℃; for 72h; Suzuki-Miyaura reaction; | 90% |
4-Bromo-1H-imidazole Specification
The 1H-Imidazole,4-bromo-, with the CAS registry number 2302-25-2, is also known as 4-Bromoimidazole. It belongs to the product categories of Blocks; Bromides; Imidazoles; Imidazol & Benzimidazole; Imidaxoles; Halogenated Heterocycles; Heterocyclic Building Blocks; ImidazolesBuilding Blocks. This chemical's molecular formula is C3H3BrN2 and molecular weight is 146.97. What's more, its systematic name is 5-bromo-1H-imidazole. It is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides.
Physical properties of 1H-Imidazole,4-bromo- are: (1)ACD/LogP: 0.97; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.9; (4)ACD/LogD (pH 7.4): 0.97; (5)ACD/BCF (pH 5.5): 2.75; (6)ACD/BCF (pH 7.4): 3.21; (7)ACD/KOC (pH 5.5): 68.59; (8)ACD/KOC (pH 7.4): 80.12; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 17.82 Å2; (13)Index of Refraction: 1.601; (14)Molar Refractivity: 26.46 cm3; (15)Molar Volume: 77.1 cm3; (16)Polarizability: 10.49×10-24cm3; (17)Surface Tension: 56.4 dyne/cm; (18)Density: 1.904 g/cm3; (19)Flash Point: 150.2 °C; (20)Enthalpy of Vaporization: 54.42 kJ/mol; (21)Boiling Point: 324.7 °C at 760 mmHg; (22)Vapour Pressure: 0.000457 mmHg at 25°C.
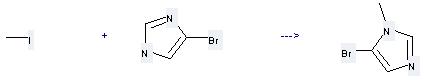
Preparation: this chemical can be prepared by 5-bromo-1-methyl-1H-imidazole at the ambient temperature. This reaction will need solvent dimethylformamide with the reaction time of 6 hours. The yield is about 66%.

Uses of of 1H-Imidazole,4-bromo-: it can be used to produce 5-bromo-1-methyl-1H-imidazole at the ambient temperature. It will need reagent N-bromosuccinimide and solvent dimethylformamide with the reaction time of 48 hours. The yeld is about 41%.
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic if swallowed. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)InChI: InChI=1S/C3H3BrN2/c4-3-1-5-2-6-3/h1-2H,(H,5,6)
(2)InChIKey: FHZALEJIENDROK-UHFFFAOYSA-N
(3)Canonical SMILES: C1=C(NC=N1)Br
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 250mg/kg (250mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: OTHER CHANGES | Toxicology and Applied Pharmacology. Vol. 89, Pg. 175, 1987. |
Related Products
- 4-Bromo-1H-imidazole
- 23022-83-5
- 2302-30-9
- 23023-13-4
- 2302-32-1
- 2302-39-8
- 23024-29-5
- 23025-68-5
- 230-26-2
- 230-27-3
- 23028-17-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View