-
Name
N,N-dimethyl-4-vinylaniline
- EINECS
- CAS No. 2039-80-7
- Article Data63
- CAS DataBase
- Density 0.959 g/cm3
- Solubility
- Melting Point 16.8 °C
- Formula C10H13N
- Boiling Point 241.6 °C at 760 mmHg
- Molecular Weight 147.22
- Flash Point 97.5 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Aniline,N,N-dimethyl-p-vinyl- (6CI,7CI,8CI);4-(Dimethylamino)styrene;4-(N,N-Dimethylamino)styrene;4-Ethenyl-N,N-dimethylaniline;4-Vinyl-N,N-dimethylaniline;N,N-Dimethyl-4-vinylaniline;N,N-Dimethyl-p-aminostyrene;N,N-Dimethyl-p-vinylaniline;NSC 3471;p-(Dimethylamino)styrene;p-(N,N-Dimethylamino)styrene;p-Vinyldimethylaniline;
- PSA 3.24000
- LogP 2.39560
Synthetic route
-
-
586-77-6
4-bromo-N,N-dimethylaniline

-
-
7486-35-3
tri-n-butyl(vinyl)tin

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); 2,8,9-tribenzyl-2,3,8,9-tetraaza-1-phosphabicyclo[3,3,3]undecane; cesium fluoride In tetrahydrofuran at 20℃; for 14h; Stille cross-coupling; | 97% |
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; cesium fluoride In 1,4-dioxane at 20℃; for 24h; Inert atmosphere; | 73% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran for 0.25h; Stage #2: 4-dimethylamino-benzaldehyde In tetrahydrofuran Wittig olefination; Further stages.; | 94% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0 - 20℃; for 2h; Wittig Olefination; Inert atmosphere; Stage #2: 4-dimethylamino-benzaldehyde In tetrahydrofuran; hexane at 0 - 20℃; for 16h; Wittig Olefination; Inert atmosphere; | 94% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.666667h; Stage #2: 4-dimethylamino-benzaldehyde In tetrahydrofuran; hexane at 25℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| With dioxathion; caesium carbonate; palladium dichloride In tetrahydrofuran; water at 85℃; for 22h; Suzuki-Miyaura reaction; | 93% |
-
-
4363-34-2
vinylboronic acid

-
-
586-77-6
4-bromo-N,N-dimethylaniline

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With cis,cis,cis-tetrakis[(diphenylphosphanyl)methyl]cyclopentane; potassium carbonate; bis(η3-allyl-μ-chloropalladium(II)) In xylene at 130℃; for 20h; Suzuki reaction; | 89% |
-
-
78-08-0
Triethoxyvinylsilane

-
-
586-77-6
4-bromo-N,N-dimethylaniline

-
A
-
7478-69-5
1,1-Bisethylene

-
B
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
C
-
22210-79-3
(E)-1,2-bis-[4-(dimethylamino)phenyl]ethene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; poly(ethylene glycol) 2000; palladium diacetate In water at 150℃; for 2h; Hiyama reaction; | A n/a B 86% C n/a |

-
-
78-08-0
Triethoxyvinylsilane

-
-
586-77-6
4-bromo-N,N-dimethylaniline

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| Stage #1: Triethoxyvinylsilane With sodium hydroxide In water at 20℃; for 0.0833333h; Sealed tube; Stage #2: 4-bromo-N,N-dimethylaniline With palladium diacetate In water at 140℃; for 3h; Reagent/catalyst; Sealed tube; | 86% |
-
-
71089-15-1
1-(4-N,N-dimethylaminophenyl)propane

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With [CuCl(ClIPr)]; sodium t-butanolate; tert-butyl alcohol In tetrahydrofuran; hexane at 40℃; for 20h; Inert atmosphere; chemoselective reaction; | 82% |
-
-
698-70-4
4-Iodo-N,N-dimethylaniline

-
-
1826-67-1
vinyl magnesium bromide

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| Stage #1: vinyl magnesium bromide With gallium(III) trichloride In tetrahydrofuran; hexane; dimethyl sulfoxide at 25℃; Stage #2: 4-Iodo-N,N-dimethylaniline With tris-(o-tolyl)phosphine; tris(dibenzylideneacetone)dipalladium(0) chloroform complex In tetrahydrofuran; hexane; dimethyl sulfoxide Heating; | 81% |
-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With (IMes)CuCl; triphenylphosphine; isopropyl alcohol In 1,4-dioxane; diethyl ether at 60℃; for 16h; | 81% |
-
-
27200-84-6
methyl triphenylphosphonium bromide

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 22℃; for 18h; Inert atmosphere; | 78% |
| With n-butyllithium 1) hexane, ether, 4 h, r.t. 2) 25 deg C, ether or benzene; Yield given. Multistep reaction; | |
| With n-butyllithium 1.) diethyl ether, hexane, 4 h, room temp., 2.) 12 h, reflux; Yield given. Multistep reaction; |
-
-
2065-66-9
methyl-triphenylphosphonium iodide

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide for 3.5h; Ambient temperature; | 71% |
| Stage #1: methyl-triphenylphosphonium iodide With n-butyllithium In tetrahydrofuran; hexane for 0.5h; Stage #2: 4-dimethylamino-benzaldehyde In tetrahydrofuran; hexane for 2h; |
-
-
3112-85-4
Methyl phenyl sulfone

-
-
1703-46-4
N,N-dimethyl-4-hydroxymethylaniline

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With sodium hydride In mineral oil at 135℃; for 17h; Inert atmosphere; Schlenk technique; | 60% |

| Conditions | Yield |
|---|---|
| With phenylsilane In N,N-dimethyl acetamide at 60℃; for 2h; Sealed tube; | 45% |
| With proazaphosphatrane; 9-bora-bicyclo[3.3.1]nonane In tetrahydrofuran at 90℃; under 750.075 Torr; for 1h; Inert atmosphere; Schlenk technique; | 33% |
-
-
7144-49-2
2-methanesulfonylbenzothiazole

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran; hexane -78 deg C, 3 h then rt., 1 h; | 44% |

| Conditions | Yield |
|---|---|
| With phenylsilane In N,N-dimethyl-formamide at 90℃; under 760.051 Torr; for 24h; Schlenk technique; | 38% |
-
-
124-38-9
carbon dioxide

-
-
99-92-3
4-Aminoacetophenone

-
A
-
34551-44-5
N-(4-vinylphenyl)formamide

-
B
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
C
-
4150-37-2
N,N-dimethyl-4-ethylaniline

| Conditions | Yield |
|---|---|
| With proazaphosphatrane; 9-bora-bicyclo[3.3.1]nonane In tetrahydrofuran at 90℃; under 750.075 Torr; for 1h; Inert atmosphere; Schlenk technique; | A 36% B 35% C 29% |

-
-
917-64-6
methyl magnesium iodide

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With diethyl ether Erhitzen des nach der Hydrolyse erhaltenen Reaktionsprodukts unter vermindertem Druck; |
-
-
75-16-1
methylmagnesium bromide

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With diethyl ether Erhitzen des nach der Hydrolyse erhaltenen Reaktionsprodukts unter vermindertem Druck; |

| Conditions | Yield |
|---|---|
| flash distillation at 150 deg C under vacuum; Yield given; |
-
-
82414-94-6
1-(4-(dimethylamino)phenyl)ethyl carbocation

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; |
-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
19493-09-5
Methylenetriphenylphosphorane

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 1h; Yield given; | |
| In tetrahydrofuran; hexane at 20℃; for 16h; Inert atmosphere; | 937 mg |
-
-
50438-76-1
Thiobenzoesaeure-O-2-(p-dimethylaminophenyl)-aethylester

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| Irradiation; |
-
-
56153-01-6
p-(N,N-dimethylamino)phenethyl bromide

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With potassium hydroxide; N,N'-diphenyl-1,4-phenylenediamine In ethanol at 60℃; |

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With silver(l) oxide und Destillieren des Reaktionsprodukts im Vakuum; |

-
-
2039-80-7
N,N-dimethy-4-vinylaniline
-
-
27389-67-9
trimethyl-(4-trimethylammonio-phenethyl)-ammonium; diiodide

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| Destillieren des Reaktionsprodukts im Vakuum; |

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| In phosphate buffer; dimethyl sulfoxide at 23℃; pH=7.4; Kinetics; |

| Conditions | Yield |
|---|---|
| Stage #1: pinacolboratamethylenetriphenylphosphonium iodide With lithium hexamethyldisilazane In N,N,N,N,N,N-hexamethylphosphoric triamide at 0℃; for 2h; Stage #2: 4-dimethylamino-benzaldehyde In N,N,N,N,N,N-hexamethylphosphoric triamide at -78 - 20℃; | 99 % Chromat. |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1) Mg / 1) diethyl ether, 1h; 2) diethyl ether, 3h 2: flash distillation at 150 deg C under vacuum View Scheme |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With potassium In tetrahydrofuran at -78℃; for 12h; | 100% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
1190375-91-7
N,N-dimethyl-4-[(1E)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)ethenyl]aniline

| Conditions | Yield |
|---|---|
| With bis(cyclopentadienyl)dihydrozirconium In toluene at 25℃; Inert atmosphere; Glovebox; | 98% |
| With tetrakis(trimethylphosphine)iron(0); norbornene In hexane at 50℃; for 18h; stereoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With copper dichloride In acetic acid at 60℃; for 6h; | 97% |
-
-
16029-98-4
trimethylsilyl iodide

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
1428963-13-6
(E)-N,N-dimethyl-4-(2-(trimethylsilyl)vinyl)aniline

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); bis(3,5-di-tert-butylphenyl)(tert-butyl)phosphine; triethylamine In 1,2-dichloro-ethane at 40℃; for 24h; Heck Reaction; Inert atmosphere; Glovebox; Sealed tube; | 96% |

| Conditions | Yield |
|---|---|
| With copper dichloride In acetic acid at 60℃; for 6h; | 96% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
115262-01-6
[bromo(difluoro)methyl](trimethyl)silane

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In toluene at 20 - 110℃; for 2h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With eosin In acetonitrile at 20℃; for 18h; Irradiation; Green chemistry; | 95% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
29333-27-5, 7342-11-2
but-3-yn-2-yl benzoate

| Conditions | Yield |
|---|---|
| RuCl2(P(C6H11)3)(1,3-dimesityl-4,5-dihydroimidazol-2-ylidene)(=CHC6H5) In benzene for 2h; Heating; | 94% |
-
-
76-09-5
2,3-dimethyl-2,3-butane diol

-
-
55718-76-8
2-chloro-1,3,2-benzodioxaborole

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
1190375-91-7
N,N-dimethyl-4-[(1E)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)ethenyl]aniline

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-1,3,2-benzodioxaborole; N,N-dimethy-4-vinylaniline With N-Methyldicyclohexylamine; (bis(3,5-di-tertbutylphenyl)(tert-butyl)phosphine)2PdCl2; lithium iodide at 70℃; for 24h; Heck Reaction; Inert atmosphere; Stage #2: 2,3-dimethyl-2,3-butane diol at 20℃; for 1h; | 94% |

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
53498-47-8
N-(4-vinyl-phenyl)-acetamide

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III) In dimethyl sulfoxide at 25℃; for 20h; Inert atmosphere; Irradiation; diastereoselective reaction; | 93% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine In hexane at 100℃; for 12h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With benzil In tetrahydrofuran for 6h; Irradiation; Inert atmosphere; Green chemistry; | 93% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; N,N,N',N'',N'''-pentamethyldiethylenetriamine; tetrabutylammomium bromide In toluene at 40℃; for 20h; Inert atmosphere; | 92% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
15164-44-0
p-(iodophenyl)carboxaldehyde

-
-
92278-49-4
4-{(E)-2-[4-(N,N-dimethylamino)phenyl]ethenyl}benzenecarbaldehyde

| Conditions | Yield |
|---|---|
| With bis(tri-t-butylphosphine)palladium(0); diisopropylamine In toluene at 80℃; for 12h; Heck Reaction; Inert atmosphere; Schlenk technique; | 92% |

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With bis(tri-t-butylphosphine)palladium(0); diisopropylamine In toluene at 80℃; for 12h; Heck Reaction; Inert atmosphere; Schlenk technique; | 92% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III) In dimethyl sulfoxide; N,N-dimethyl-formamide at 25℃; for 12h; Inert atmosphere; Irradiation; diastereoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With C10H14CoO5; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In tetrahydrofuran at 20℃; for 3h; regioselective reaction; | 90% |
-
-
51608-60-7
N-(benzylidene)-p-methylbenzenesulfonamide

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); toluene-4-sulfonamide; tricyclohexylphosphine In toluene at 100℃; Sealed tube; | 90% |

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III) In dimethyl sulfoxide at 25℃; for 20h; Inert atmosphere; Irradiation; diastereoselective reaction; | 90% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
407-25-0
trifluoroacetic anhydride

-
-
153532-02-6
(E)-4-(4-(dimethylamino)phenyl)-1,1,1-trifluorobut-3-en-2-one

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.5h; | 90% |

-
-
2039-80-7
N,N-dimethy-4-vinylaniline

-
-
73183-34-3
bis(pinacol)diborane

| Conditions | Yield |
|---|---|
| In pentane Cu-B(pinacolate) complex obtained in situ from Cu-O-t-Bu complex and B compd. (1 equiv.); reacted with alkene (1.1 equiv.) in n-pentane at room temp. for 20 min; detd. by (1)H NMR spectra; | 89% |


| Conditions | Yield |
|---|---|
| With scandium tris(ortho-N,N-dimethylaminobenzyl); trityl tetrakis(pentafluorophenyl)borate In toluene at 70℃; for 24h; regiospecific reaction; | 89% |

| Conditions | Yield |
|---|---|
| With methanol; bis(1,5-cyclooctadiene)nickel(0); (4S,4'S)-2,2'-(cyclohexane-1,1-diyl)bis(4-phenyl-4,5-dihydrooxazole); lithium ethoxide In ethanol at 50℃; for 3h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 89% |

| Conditions | Yield |
|---|---|
| With Ni(1,3-dimesitylimidazol-2-ylidene)[P(OEt)3]Br2; magnesium In tetrahydrofuran at 60℃; for 48h; Sealed tube; regioselective reaction; | 89% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; C10H14CoO5 In tetrahydrofuran at 50℃; for 6h; regioselective reaction; | 88% |
-
-
2039-80-7
N,N-dimethy-4-vinylaniline

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In benzene at 90℃; for 18h; Schlenk technique; Inert atmosphere; Glovebox; regioselective reaction; | 88% |
4-Dimethylaminostyrene Specification
The 4-Dimethylaminostyrene, with the CAS registry number 2039-80-7, is also known as p-(Dimethylamino)styrene. It belongs to the product category of Naphthyridine,Quinoline. This chemical's molecular formula is C10H13N and molecular weight is 147.22. Its IUPAC name is called 4-ethenyl-N,N-dimethylaniline.
Physical properties of 4-Dimethylaminostyrene: (1)ACD/LogP: 3.21; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.01; (4)ACD/LogD (pH 7.4): 3.2; (5)ACD/BCF (pH 5.5): 102.15; (6)ACD/BCF (pH 7.4): 159.73; (7)ACD/KOC (pH 5.5): 839.01; (8)ACD/KOC (pH 7.4): 1311.91; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Index of Refraction: 1.586; (13)Molar Refractivity: 51.49 cm3; (14)Molar Volume: 153.3 cm3; (15)Surface Tension: 35.4 dyne/cm; (16)Density: 0.959 g/cm3; (17)Flash Point: 97.5 °C; (18)Enthalpy of Vaporization: 47.86 kJ/mol; (19)Boiling Point: 241.6 °C at 760 mmHg; (20)Vapour Pressure: 0.0356 mmHg at 25°C.
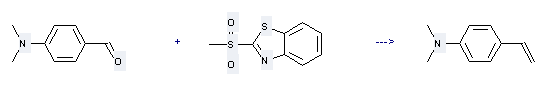
Preparation: this chemical can be prepared by 2-methanesulfonyl-benzothiazole and 4-dimethylamino-benzaldehyde. This reaction will need reagent LDA and solvent tetrahydrofuran, hexane. The reaction time is 3 hours with reaction temperature of -78 ℃. The yield is about 44%.
Uses of 4-Dimethylaminostyrene: it can be used to produce trans-1,2-bis[p-(dimethylamino)phenyl]cyclobutane at ambient temperature. This reaction will need reagent Fe(NO3)3 and solvent methanol with reaction time of 1.5 hours. The yield is about 81%.

You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CN(C)C1=CC=C(C=C1)C=C
(2)InChI: InChI=1S/C10H13N/c1-4-9-5-7-10(8-6-9)11(2)3/h4-8H,1H2,2-3H3
(3)InChIKey: GQWAOUOHRMHSHL-UHFFFAOYSA-N
Related Products
- 4-Dimethylaminostyrene
- 2039-82-9
- 203983-14-6
- 20398-34-9
- 2039-85-2
- 2039-86-3
- 2039-87-4
- 2039-88-5
- 2039-89-6
- 2039-96-5
- 2040-01-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View