-
Name
Ferulic Acid
- EINECS 214-490-0
- CAS No. 1135-24-6
- Article Data223
- CAS DataBase
- Density 1.316 g/cm3
- Solubility Soluble in water
- Melting Point 168-172 °C(lit.)
- Formula C10H10O4
- Boiling Point 372.3 °C at 760 mmHg
- Molecular Weight 194.187
- Flash Point 150.5 °C
- Transport Information
- Appearance Pale Yellow Solid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-;(E)-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enoate;3-(4-hydroxy-3-methoxy-phenyl)prop-2-enoic acid;3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid;caffeic acid 3-methyl ether;3-(4-Hydroxy-3-methoxyphenyl)acrylic acid;2-Propenoic acid,3-(4-hydroxy-3-methoxyphenyl)-;2-Propenoic acid, 3- (4-hydroxy-3-methoxyphenyl)-;Ferulate;3-Methoxy-4-hydroxy-trans-cinnamate;Cinnamic acid, 4-hydroxy-3-methoxy-;3-methoxy-4-hydroxycinnamic acid;Natural ferulic acid;Ferulic acid 99.5%;FerulicAcid;Gamma-Oryzanol, Ferulic acid;Fumalic acid;
- PSA 66.76000
- LogP 1.49860
Synthetic route
-
-
4046-02-0
ethyl 4-hydroxy-3-methoxycinnamate

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water for 3h; Reflux; | 100% |
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 8h; | 98% |
| With Sporotrichum thermophile type C feruloyl esterase; water Enzyme kinetics; | |
| With recombinant Tan410 In aq. phosphate buffer at 35℃; for 0.75h; pH=7; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With piperidine; pyridine for 0.0833333h; microwave irradiation; | 94% |
| With piperidine; p-toluidine In toluene at 80℃; for 3h; Knoevenagel Condensation; | 94% |
| With bismuth(III) chloride for 0.0833333h; Doebner condensation; Microwave irradiation; Neat (no solvent); | 93% |

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| bismuth(lll) trifluoromethanesulfonate In tetrahydrofuran; water at 20℃; for 0.416667h; | 90% |

| Conditions | Yield |
|---|---|
| With lithium hydroxide monohydrate In tetrahydrofuran; methanol; water at 20℃; for 18h; Inert atmosphere; Schlenk technique; Glovebox; | 89% |
| With Sporotrichum thermophile type C feruloyl esterase; water Enzyme kinetics; |
-
-
141-82-2
malonic acid

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide at 60℃; for 8h; Knoevenagel-Doebner condensation; | 83% |
-
-
98885-93-9, 115851-80-4, 147556-16-9
curcumin

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| With 5,10,15,20-tetra(2',6'-dichlorophenyl)porphyrinatoiron(III) chloride; dihydrogen peroxide; acetylacetone In dichloromethane at 20℃; for 2.5h; | A 2.1% B 3.3% C 70% |


-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With copper(I) oxide; 1-D-O-Methyl-chiro-inositol; sodium hydroxide In water at 120℃; for 6h; | 60% |
-
-
85364-70-1
3-methyl-4-nitro-5-<2-(3-methoxy-4-hydroxyphenyl)ethenyl>isoxazole

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 2h; Heating; | 49.7% |

| Conditions | Yield |
|---|---|
| O-methyltransferase pH 7; |
-
-
63644-62-2, 123821-69-2, 62706-35-8
(E,E)-3-(4-hydroxy-3-methoxyphenyl)allyl 3-(4-hydroxy-3-methoxyphenyl)acrylate

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
458-35-5, 32811-40-8, 69056-21-9
coniferal alcohol

-
C
-
63644-71-3
(E)-4-(3-methoxy-1-propenyl)-2-methoxyphenol

| Conditions | Yield |
|---|---|
| Product distribution; hydrolysis by various reaction conditions to various product; |

-
-
2596-47-6, 147677-05-2, 34749-55-8
(E)-3-(4-acetoxy-3-methoxyphenyl)acrylic acid

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 4h; Heating; |
-
-
131623-14-8
6'-O-trans-feruloylnodakenin

-
A
-
2280-44-6
D-Glucose

-
B
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
C
-
495-32-9
(+)-marmesin

| Conditions | Yield |
|---|---|
| In sulfuric acid for 0.666667h; Heating; | |
| With sulfuric acid for 0.666667h; Heating; |

-
-
101330-61-4
di-(E)-feruloylspermidine

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 100℃; for 4h; |
-
-
96014-22-1
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide

-
A
-
61-54-1
tryptamine

-
B
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| In sodium hydroxide |
-
-
83608-86-0, 78510-19-7
N-trans-feruloyl-3-O-methyldopamine

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
554-52-9
3-Methoxytyramine

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
117405-51-3
4-hydroxy-3-methoxycinnamic acid 4-O-β-D-glucopyranoside

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
50-99-7
D-glucose

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol for 3h; Heating; | A 21 mg B n/a |
-
-
22608-11-3, 24939-17-1, 33171-16-3
demethoxycurcumin

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
7400-08-0
p-Coumaric Acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 2h; Product distribution; Heating; |

-
A
-
58-86-6
D-xylose

-
B
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
C
-
5328-37-0
L-arabinose

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aspergillus niger "cellulase" Product distribution; |

-
-
85011-57-0
scopoletin 7-O-(6-O-feruloyl-β-D-glucopyranoside)

-
A
-
2280-44-6
D-Glucose

-
B
-
92-61-5
7-hydroxy-6-methoxy-2H-1-benzopyran-2-one

-
C
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 6h; Heating; water bath; |

-
-
85011-57-0
scopoletin 7-O-(6-O-feruloyl-β-D-glucopyranoside)

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
531-44-2
scopolin

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 60℃; for 0.583333h; |
-
-
84873-15-4
(2E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid 2-(4-hydroxyphenyl)ethyl ester

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; ethanol for 0.166667h; | A 488 mg B 530 mg |
-
-
84976-26-1
O-<5-O-(trans-feruloyl)-α-L-arabinofuranosyl>-(1<*>3)-O-β-D-xylopyranosyl-(1<*>4)-D-xylopyranose

-
A
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
B
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
C
-
7296-56-2
β-L-arabinopyranose

| Conditions | Yield |
|---|---|
| With hydrolysis with cellulase or trifluoroacetic acid Product distribution; |

-
-
84976-26-1
O-<5-O-(trans-feruloyl)-α-L-arabinofuranosyl>-(1<*>3)-O-β-D-xylopyranosyl-(1<*>4)-D-xylopyranose

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
141923-44-6
α-L-arabinofuranosyl-(1→3)-β-D-xylopyranosyl-(1→4)-D-xylopyranose

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 24h; Ambient temperature; |
-
-
80159-08-6
(E)-6-O-feruloylscandoside methyl ester

-
A
-
18842-99-4
deacetylasperulosidic acid

-
B
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With barium dihydroxide Ambient temperature; Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 3h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 100℃; for 4h; Title compound not separated from byproducts; |

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
22336-76-1
3-camphanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 24h; Heating; | A 30 mg B 10 mg |

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 90℃; for 0.5h; |

-
A
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
98941-66-3
angrendiol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 8h; Ambient temperature; | A 206 mg B 223 mg |
-
-
823788-11-0
(E)-methyl 3-(3-methoxy-4-((methylsulfonyl)oxy)phenyl)acrylate

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 1h; Heating; |

| Conditions | Yield |
|---|---|
| With Dowex 50 W x 8200-400 Heating; | 100% |
| With thionyl chloride Ambient temperature; | 100% |
| With sulfuric acid at 0℃; for 12h; Darkness; Reflux; | 99% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
64-17-5
ethanol

-
-
4046-02-0
ethyl 4-hydroxy-3-methoxycinnamate

| Conditions | Yield |
|---|---|
| With acetyl chloride at 20℃; | 100% |
| With sulfuric acid at 88℃; under 1034.32 Torr; for 0.05h; Microwave irradiation; | 94% |
| With sulfuric acid for 4h; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| With phosphate buffer; Catharanthus roseus at 25℃; for 72h; pH=6.0; | 100% |
| With 4-methoxy-phenol In water; N,N-dimethyl-formamide at 150℃; for 4h; | 94% |
| With sodium hydroxide In water at 30℃; for 3h; pH=8.5; Microbiological reaction; | 93.1% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
1135-23-5
3-(4-hydroxy-3-methoxyphenyl)propionic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate under 760 Torr; | 100% |
| With hydrogen; palladium on activated charcoal In ethanol at 20℃; under 2068.59 Torr; | 100% |
| With hydrogen; palladium on activated charcoal In methanol; ethyl acetate under 2068.59 Torr; for 5h; | 100% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
108-24-7
acetic anhydride

-
-
2596-47-6, 147677-05-2, 34749-55-8
(E)-3-(4-acetoxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With pyridine at 37℃; Inert atmosphere; | 100% |
| With chloro-trimethyl-silane; sodium iodide at 20℃; for 0.333333h; Reagent/catalyst; | 96% |
| With sodium hydroxide In water at 20℃; for 1.5h; Cooling with ice; | 95.35% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
77-78-1
dimethyl sulfate

-
-
5396-64-5
methyl (E)-3-(3,4-dimethoxyphenyl)acrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 100% |
| With potassium carbonate In acetone for 3h; Reflux; | 90% |
| With potassium carbonate In acetone for 36h; | 4.2 g |
| With potassium carbonate In acetone |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
100-39-0
benzyl bromide

-
-
94475-61-3
(E)-3-(3-methoxy-4-benzyloxyphenyl)acrylic acid benzyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 48h; Esterification; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 4h; | 91% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
55947-32-5
(E)-3-(3-Methoxy-4-trimethylsilanyloxy-phenyl)-acrylic acid

| Conditions | Yield |
|---|---|
| 100% |
-
-
50-67-9
3-(2-aminoethyl)-1H-indol-5-ol

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
68573-23-9
(2E)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-3-(4-hydroxy-3-methoxyphenyl)-2-propenamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide | 99% |
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 3-(2-aminoethyl)-1H-indol-5-ol With triethylamine In dichloromethane; N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 57% |
| Stage #1: 3-(2-aminoethyl)-1H-indol-5-ol; (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With pyridine; dicyclohexyl-carbodiimide at 20℃; for 24h; Stage #2: With potassium hydroxide In methanol at 20℃; for 4h; |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With sodium In ethanol Reflux; Inert atmosphere; | 99% |
| With sodium hydroxide In methanol | 82% |
| With sodium hydroxide In water Heating; |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine at 0 - 20℃; Inert atmosphere; | 99% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
458-35-5, 32811-40-8, 69056-21-9
coniferal alcohol

| Conditions | Yield |
|---|---|
| With ATP; NADPH; magnesium chloride In aq. phosphate buffer at 30℃; for 18h; pH=7.5; Green chemistry; Enzymatic reaction; | 98% |
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With chloroformic acid ethyl ester; triethylamine In 1,4-dioxane for 1h; Inert atmosphere; Stage #2: With sodium tetrahydroborate In 1,4-dioxane; N,N-dimethyl-formamide for 1h; Inert atmosphere; | 33% |
| Multi-step reaction with 2 steps 1: 96 percent / HCl / 48 h / 20 °C 2: 87 percent / LiAlH4 / diethyl ether / 12 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With tricopper(II) bis(benzene-1,3,5-tricarboxylate) trihydrate; dihydrogen peroxide In ethanol; water; acetonitrile at 100℃; for 1h; | 98% |
| With dihydrogen peroxide In ethanol; water; acetonitrile for 4h; Mechanism; Kinetics; Reflux; | 71% |
| titanium(IV) oxide In water at 65℃; under 3.75038 Torr; Irradiation; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| at 85℃; for 0.05h; Microwave irradiation; | 98% |
-
-
3731-51-9
(2-aminomethylpyridine)

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
113985-17-4
(E)-3-(4-hydroxy-3-methoxyphenyl)-N-(pyridin-2-ylmethyl)acrylamide

| Conditions | Yield |
|---|---|
| at 85℃; for 0.05h; Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| at 85℃; for 0.05h; Microwave irradiation; | 98% |
-
-
71-23-8
propan-1-ol

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
59831-93-5
propyl (2E)-3-(4-hydroxy-3-methoxyphenyl)-prop-2-enoate

| Conditions | Yield |
|---|---|
| With sulfuric acid for 6h; Reflux; | 97% |
| With sulfuric acid at 107℃; under 1034.32 Torr; for 0.0666667h; Microwave irradiation; | 94% |
| With acetyl chloride for 48h; | 90% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
62-53-3
aniline

-
-
55882-80-9
3-(4-hydroxy-3-methoxyphenyl)-N-(4-phenyl)prop-(2E)-enamide

| Conditions | Yield |
|---|---|
| at 125℃; for 0.0833333h; Microwave irradiation; | 97% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 8h; | 76% |
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran for 18h; | 44% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 25℃; Inert atmosphere; | 33% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
109-76-2
Trimethylenediamine

-
-
157771-76-1
N,N'-(trimethylene)-bis[(E)-3-(4-hydroxy-3-methoxyphenyl)acrylamide]

| Conditions | Yield |
|---|---|
| at 110℃; for 0.116667h; Microwave irradiation; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide for 0.5h; Stage #2: 4-ethoxycarbonylpiperazine In N,N-dimethyl-formamide at 20℃; | 96.7% |

| Conditions | Yield |
|---|---|
| With isopropyl alcohol; palladium on activated charcoal Product distribution; Heating; | 96% |
| With isopropyl alcohol; palladium on activated charcoal Heating; | 96% |
| Multi-step reaction with 2 steps 1: palladium/calcium carbonate; methanol / Hydrogenation 2: soda lime / 280 °C / 20 Torr View Scheme |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
106-95-6
allyl bromide

-
-
115375-24-1
(E)-3-(4-(allyloxy)-3-methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid; allyl bromide With potassium carbonate In acetonitrile at 70℃; for 18h; Inert atmosphere; Stage #2: With potassium hydroxide In methanol; water; acetonitrile at 660℃; for 24h; Inert atmosphere; | 96% |
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With potassium carbonate In acetone at 20℃; for 0.5h; Stage #2: allyl bromide In acetone for 48h; Williamson ether synthesis; Reflux; Stage #3: With sodium hydroxide In ethanol for 2h; Reflux; | 84% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 3.5h; | 96% |

| Conditions | Yield |
|---|---|
| at 85℃; for 0.05h; Microwave irradiation; | 96% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
15356-60-2
(1S,2R,5S)-(+)-menthol

| Conditions | Yield |
|---|---|
| With sulfuric acid-immobilized mesoporous silica catalyst at 70℃; for 1.5h; Green chemistry; | 96% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
474398-83-9
(2E)-3-(4-hydroxy-3-methoxyphenyl)acrylohydrazide

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetonitrile at 25℃; for 2h; Stage #2: With hydrazine hydrate In acetonitrile at 0 - 10℃; | 96% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
854737-54-5
(E)‐3‐(4‐((tert‐butyldimethylsilyl)oxy)‐3‐methoxyphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 25℃; for 14h; | 95% |
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With 1H-imidazole In N,N-dimethyl-formamide Stage #2: tert-butyldimethylsilyl chloride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #3: With potassium carbonate In tetrahydrofuran; methanol; water for 1h; | 93% |
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; | 67% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
86945-25-7
4-(2-aminoethyl)-1-(phenylmethyl)piperidine

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran at 120℃; for 0.116667h; Microwave irradiation; Inert atmosphere; Sealed tube; Stage #2: 4-(2-aminoethyl)-1-(phenylmethyl)piperidine In tetrahydrofuran at 120℃; for 0.166667h; Microwave irradiation; Inert atmosphere; Sealed tube; | 95% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 12h; | 75% |
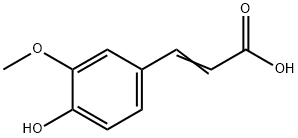
4-Hydroxy-3-methoxycinnamic acid Chemical Properties
IUPAC Name: (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid
Empirical Formula: C10H10O4
Molecular Weight: 194.184g/mol
EINECS: 208-679-7
Structure of 4-Hydroxy-3-methoxycinnamic acid (CAS NO.1135-24-6):
Index of Refraction: 1.626
Molar Refractivity: 52.26 cm3
Molar Volume: 147.4 cm3
Polarizability: 20.72×10-24cm3
Surface Tension: 56.1 dyne/cm
Density: 1.316 g/cm3
Flash Point: 150.5 °C
Enthalpy of Vaporization: 65.35 kJ/mol
Melting Point: 168-172 °C(lit.)
Boiling Point: 372.3 °C at 760 mmHg
Vapour Pressure: 3.34E-06 mmHg at 25°C
Water Solubility: soluble
Product Categories: FINE Chemical & INTERMEDIATES;Aromatic Cinnamic Acids, Esters and Derivatives;Aromatics
Canonical SMILES: COC1=C(C=CC(=C1)C=CC(=O)O)O
Isomeric SMILES: COC1=C(C=CC(=C1)/C=C/C(=O)O)O
InChI: InChI=1S/C10H10O4/c1-14-9-6-7(2-4-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+
InChIKey: KSEBMYQBYZTDHS-HWKANZROSA-N
4-Hydroxy-3-methoxycinnamic acid Uses
1.It is an antioxidant in the sense that it is reactive toward free radicals such as reactive oxygen species (ROS). ROS and free radicals are implicated in DNA damage, cancer, accelerated cell aging.
2.It may have pro-apoptotic effects in cancer cells, thereby leading to their destruction.
3.It may be effective at preventing cancer induced by exposure to the carcinogenic compounds benzopyreneand 4-nitroquinoline 1-oxide.
4-Hydroxy-3-methoxycinnamic acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD | intraperitoneal | > 350mg/kg (350mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: ATAXIA BEHAVIORAL: REGIDITY | Indian Journal of Pharmaceutical Sciences. Vol. 49, Pg. 77, 1987. |
| mouse | LD50 | intravenous | 857mg/kg (857mg/kg) | Chemical and Pharmaceutical Bulletin. Vol. 38, Pg. 1620, 1990. |
4-Hydroxy-3-methoxycinnamic acid Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 3
RTECS: UD3365500
Hazard Note: Irritant
Hazardous Substances Data: 1135-24-6(Hazardous Substances Data)
4-Hydroxy-3-methoxycinnamic acid Specification
4-Hydroxy-3-methoxycinnamic acid , its cas register number is 1135-24-6. It also can be called 3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid ; 3-Methoxy-4-hydroxycinnamic acid ; 4-Hydroxy-3-methoxy cinnamic acid ; CCRIS 3256 ; Cinnamic acid, 4-hydroxy-3-methoxy- ; Coniferic acid ; Ferulic acid .
4-Hydroxy-3-methoxycinnamic acid (CAS NO.1135-24-6) is a pale yellow solid.
Related Products
- 4-Hydroxy-3-methoxycinnamic acid
- 113525-88-5
- 1135-26-8
- 1135283-08-7
- 1135283-53-2
- 113-53-1
- 1135324-00-3
- 113534-16-0
- 113534-17-1
- 113538-22-0
- 113538-80-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View