-
Name
4-N-OCTYLPHENOL
- EINECS 217-302-5
- CAS No. 1806-26-4
- Article Data27
- CAS DataBase
- Density 0.961 g/mL at 25 °C(lit.)
-
Solubility
- Hazard Symbols 8 UN NO.
- Synonyms Phenol,p-octyl- (6CI,7CI,8CI);4-Octylphenol;4-n-Octylphenol;p-(n-Octyl)phenol;p-Octylphenol;
- PSA 20.23000
- LogP 4.29520
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; mercury; zinc | 78% |
| With hydrogenchloride; mercury; zinc | 70% |
| With hydrogenchloride; amalgamated zinc; acetic acid |

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; magnesium(II) acetate tetrahydrate; 4,4'-di-tert-butyl-2,2'-bipyridine In N,N-dimethyl acetamide at 40℃; for 3h; Schlenk technique; Inert atmosphere; regioselective reaction; | 53% |
-
-
62170-25-6
1-(4-methoxyphenyl)octan-1-one

-
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrazine hydrate; phenol weiteres Reagens: Diaethylenglykol. Erhitzen des Reaktionsprodukts mit wss. HI, Essigsaeure und Acetanhydrid; |

| Conditions | Yield |
|---|---|
| With hydrogen iodide; acetic acid |
-
-
124-13-0
Octanal

-
-
108-95-2
phenol

-
A
-
1233-26-7
1,1-bis(4-hydroxyphenyl)octane

-
B
-
949-13-3
2-octylphenol

-
C
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With Al3+-exchanged montmorillonite at 100℃; for 48h; Product distribution; Mechanism; other aldehydes and ketones; also AlCl3; |


| Conditions | Yield |
|---|---|
| In water at 25℃; for 7h; Irradiation; Yields of byproduct given. Title compound not separated from byproducts; |
-
-
124-13-0
Octanal

-
-
108-95-2
phenol

-
A
-
1233-26-7
1,1-bis(4-hydroxyphenyl)octane

-
B
-
949-13-3
2-octylphenol

-
C
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With Al(3+)-montmorillonite at 100℃; for 48h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
124-13-0
Octanal

-
-
108-95-2
phenol

-
A
-
1233-26-7
1,1-bis(4-hydroxyphenyl)octane

-
B
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With Al(3+)-montmorillonite at 100℃; for 48h; Yield given. Further byproducts given. Yields of byproduct given; | |
| With Al(3+)-montmorillonite at 100℃; for 48h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; cetyltrimethylammonim bromide In water at 25℃; Kinetics; |
-
-
16245-79-7
4-octylaniline

-
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) NaNO2, 3M aq. HCl, 2.) NaOAc, Na2SO3 / 1.) H2O, 0 deg C, 20 min, 2.) H2O, 0 deg C, 16 h 2: H2O / 7 h / 25 °C / Irradiation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AlCl3 / nitrobenzene / 0.5 h / 50 °C 2: Zn-Hg, 5 N HCl / ethanol / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 57 percent / AlCl3 / nitrobenzene / 8 h / Ambient temperature 2: 78 percent / Zn(Hg), HCl View Scheme | |
| Multi-step reaction with 2 steps 1: AlCl3 / CH2Cl2 / 1.) 0 deg C, 30 min; 0 deg C, 30 min; 2.) room temperature, overnight 2: 1.) hydrazine hydrate, 2.) KOH / 1.) diethylene glycol, reflux, 1h; 2.) reflux, 1h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AlCl3 / nitrobenzene / 0.5 h / 50 °C 2: Zn-Hg, 5 N HCl / ethanol / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 86 percent / SOCl2 2: 60 percent / AlCl3 / nitrobenzene 3: 70 percent / Zn(Hg), HCl View Scheme | |
| Multi-step reaction with 5 steps 1: thionyl chloride / 60 °C / Reflux 2: 70 - 75 °C 3: aluminum (III) chloride / 3.5 h / 20 - 100 °C 4: hydrazine hydrate / diethylene glycol / 2 h / Reflux 5: potassium hydroxide / diethylene glycol / 5 h / 20 - 200 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / AlCl3 / nitrobenzene 2: 70 percent / Zn(Hg), HCl View Scheme | |
| Multi-step reaction with 3 steps 1: aluminum (III) chloride / 3.5 h / 20 - 100 °C 2: hydrazine hydrate / diethylene glycol / 2 h / Reflux 3: potassium hydroxide / diethylene glycol / 5 h / 20 - 200 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 86 percent / SOCl2 2: 60 percent / AlCl3 / nitrobenzene 3: 70 percent / Zn(Hg), HCl View Scheme | |
| Multi-step reaction with 2 steps 1: 57 percent / AlCl3 / nitrobenzene / 8 h / Ambient temperature 2: 78 percent / Zn(Hg), HCl View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AlCl3 / CH2Cl2 / 1.) 0 deg C, 30 min; 0 deg C, 30 min; 2.) room temperature, overnight 2: 1.) hydrazine hydrate, 2.) KOH / 1.) diethylene glycol, reflux, 1h; 2.) reflux, 1h View Scheme | |
| Multi-step reaction with 2 steps 1: AlCl3 / 100 - 130 °C 2: amalgamated zinc; concentrated aqueous HCl; glacial acetic acid View Scheme | |
| Multi-step reaction with 2 steps 1: AlCl3 / 100 - 130 °C 2: amalgamated zinc; concentrated aqueous HCl; glacial acetic acid View Scheme | |
| Multi-step reaction with 2 steps 1: zuletzt bei 100grad, und Erwaermen des Reaktionsprodukts mit AlCl3 in Nitrobenzol auf 75grad 2: amalgamated zinc; diluted aqueous HCl / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1,1,2,2-tetrachloro-ethane; AlCl3 2: N2H4+H2O; KOH; phenol / weiteres Reagens: Diaethylenglykol. Erhitzen des Reaktionsprodukts mit wss. HI, Essigsaeure und Acetanhydrid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: HI, AcOH View Scheme |
-
-
1089700-47-9
C14H22N2O

-
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In diethylene glycol at 20 - 200℃; for 5h; |
-
-
111-66-0
oct-1-ene

-
-
108-95-2
phenol

-
A
-
1818-07-1
octyloxybenzene

-
B
-
949-13-3
2-octylphenol

-
C
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With porous aromatic framework modified by aluminum chloride at 90℃; for 6h; Reagent/catalyst; Temperature; Friedel-Crafts Alkylation; Autoclave; |


-
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; acetic acid at 80℃; under 7500.75 Torr; for 2h; Pressure; Reagent/catalyst; Autoclave; |
-
-
2969-81-5
4-bromoethylbutanoate

-
-
1806-26-4
p-Octylphenol

-
-
1071001-73-4
4-(4-octylphenoxy)butyric acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone for 18h; Reflux; | 100% |
| With potassium carbonate In acetone Reflux; | 71% |
| With potassium carbonate In acetone for 24h; Heating / reflux; | 71% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide at 20℃; for 5h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: p-Octylphenol With pyridine In dichloromethane at -10℃; for 0.0833333h; Inert atmosphere; Stage #2: trifluoromethanesulfonic anhydride In dichloromethane Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 5h; Reflux; | 98% |
| With potassium carbonate In acetone for 5h; Reflux; | 98% |
-
-
1806-26-4
p-Octylphenol

-
-
16420-13-6
N,N-Dimethylthiocarbamoyl chloride

-
-
869650-51-1
O-p-octylphenyl N,N-dimethylthiocarbamate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 85℃; | 93% |
-
-
1806-26-4
p-Octylphenol

-
-
124482-92-4
2-cyanoethyl-N,N-(diisopropylamino)chlorophosphine

-
-
1275594-48-3
C23H39N2O2P

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 0.5h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| In ethanol; water for 48h; Reflux; | 90% |

-
-
1806-26-4
p-Octylphenol

-
-
1220974-10-6
2-iodo-4-octylphenol

| Conditions | Yield |
|---|---|
| With iodine; silver trifluoroacetate In dichloromethane at 0 - 20℃; | 87% |
| With iodine; silver trifluoroacetate at 0 - 20℃; for 1h; | 87% |
-
-
741606-45-1
1-oxiranylmethylindole-5-carbonitrile

-
-
1806-26-4
p-Octylphenol

-
-
741606-27-9
1-[2-hydroxy-3-(4-octylphenoxy)propyl]indole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 60℃; for 2h; | 85% |

| Conditions | Yield |
|---|---|
| 85% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; Sealed tube; | 85% |
-
-
1806-26-4
p-Octylphenol

-
-
60456-23-7
(S)-oxiranemethanol

-
-
925706-87-2
(R)-2-(4-octylphenoxymethyl)oxirane

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; for 24h; Mitsunobu reaction; | 84% |
-
-
1806-26-4
p-Octylphenol

-
-
1849-02-1
2-chloro-1-methyl-benzimidazole

-
-
78765-43-2
1-Methyl-2-(4-octyl-phenoxy)-1H-benzoimidazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 24h; Heating; | 83% |
-
-
1806-26-4
p-Octylphenol

-
-
24777-05-7
2-Nitro-4-octyl-phenol

| Conditions | Yield |
|---|---|
| With nitric acid; acetic acid In chloroform for 1.5h; Ambient temperature; | 82% |
| With NaNO3; sulfuric acid In hexane; water; ethyl acetate | 68% |
| With nitric acid; acetic acid |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 1h; | 81% |
-
-
1026660-75-2
di-tert-butyl 5-(oxiran-2-ylmethoxy)indole-1,2-carboxylate

-
-
1806-26-4
p-Octylphenol

-
-
1026660-76-3
di-tert-butyl 5-[2-hydroxy-3-(4-octylphenoxy)propoxy]indole-1,2-carboxylate

| Conditions | Yield |
|---|---|
| With dmap at 40℃; for 48h; | 81% |
-
-
1806-26-4
p-Octylphenol

-
-
205647-74-1
(R)-tert-butyl 2,2-dimethyl-4-[(tosyloxy)methyl]-oxazolidine-3-carboxylate

-
-
1643461-35-1
(S)-tert-butyl 2,2-dimethyl-4-[(4-octylphenoxy)methyl]oxazolidine-3-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: p-Octylphenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.5h; Stage #2: (R)-tert-butyl 2,2-dimethyl-4-[(tosyloxy)methyl]-oxazolidine-3-carboxylate In N,N-dimethyl-formamide; mineral oil at 70℃; for 3h; | 81% |
-
-
1806-26-4
p-Octylphenol

-
-
57835-36-6
2-bromo-4-octylphenol

| Conditions | Yield |
|---|---|
| With bromine; sodium hydrogencarbonate In chloroform at 0℃; | 80% |
| With bromine; 1,2-dichloro-ethane |
-
-
1806-26-4
p-Octylphenol

-
-
57044-25-4
(R)-oxiranemethanol

-
-
925706-88-3
(S)-2-(4-octylphenoxymethyl)oxirane

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; for 24h; Mitsunobu reaction; | 80% |

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 20h; | 78% |

-
-
376642-29-4
3-[2-(4-benzyloxyphenyl)-5-methyl-1H-pyrrol-1-yl]propan-1-ol

-
-
1806-26-4
p-Octylphenol

-
-
10465-81-3
1,1'-(Azodicarbonyl)dipiperidin

-
-
376642-30-7
2-(4-benzyloxyphenyl)-5-methyl-1-[3-(4-octyloxyphenyl)propyl]-1H-pyrrole

| Conditions | Yield |
|---|---|
| With triphenylphosphine In toluene | 77.4% |

-
-
1806-26-4
p-Octylphenol

-
-
107-30-2
chloromethyl methyl ether

-
-
123914-95-4
1-methoxymethoxy-4-octyl-benzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In dichloromethane; water at -10℃; for 0.5h; | 74% |
-
-
1806-26-4
p-Octylphenol

-
-
1452822-15-9
C21H23BrF4O

-
-
1452822-24-0
(Z)-4-(hexyloxy)-4'-(2,3,3-trifluoro-3-(4-octylphenoxy)prop-1-enyl)biphenyl

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 80℃; Schlenk technique; Inert atmosphere; stereoselective reaction; | 74% |
-
-
22094-18-4
1,3-dibromo-2,2-dimethoxypropane

-
-
1806-26-4
p-Octylphenol

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In N,N-dimethyl-formamide for 10h; Reflux; | 74% |
-
-
1806-26-4
p-Octylphenol

-
-
1586018-38-3
tert-butyl 1-(oxiran-2-ylmethyl)indazole-5-carboxylate

-
-
1586018-39-4
tert-butyl 1-[2-hydroxy-3-(4-octylphenoxy)propyl]indazole-5-carboxylate

| Conditions | Yield |
|---|---|
| With dmap at 120℃; for 2h; | 73% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid for 0.333333h; | 71% |
-
-
1806-26-4
p-Octylphenol

-
-
24801-88-5
3-(triethoxypropyl) isocyanate

-
-
1314704-83-0
4-octylphenyl-3-(triethoxysilyl)propylcarbamate

| Conditions | Yield |
|---|---|
| With DBDU In tetrahydrofuran at 75℃; for 24h; | 71% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; formamide In 1,4-dioxane at 95℃; for 24h; | 70% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; | 70% |
4-OCTYLPHENOL Chemical Properties
MF: C14H22O
MW: 206.32
EINECS: 217-302-5
mp: 44-45 °C(lit.)
bp: 150 °C4 mm Hg(lit.)
density: 0.961 g/mL at 25 °C(lit.)
Fp: >230 °F
CAS DataBase Reference: 1806-26-4(CAS DataBase Reference)
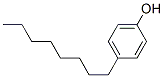
The structure of 4-OCTYLPHENOL is:
4-OCTYLPHENOL Toxicity Data With Reference
1、RTECS#: CAS# 1806-26-4: SM5787000
2、LD50/LC50: RTECS: Not available.
3、Carcinogenicity: 4-Octylphenol - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
4、Other: The toxicological properties have not been fully investigated. See actual entry in RTECS for complete information.
4-OCTYLPHENOL Consensus Reports
4-OCTYLPHENOL Safety Profile
Hazard Codes: Xi
RIDADR: UN 2430 8/PG 2
WGK Germany: 3
HazardClass: 8
Risk Statements: Irritating to the eyes;Irritating to the skin
Safety Statements: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;Wear suitable protective clothing
PackingGroup: III Experimental reproductive effects.
When heated to decomposition it emits acrid smoke and irritating vapors.
4-OCTYLPHENOL Specification
1、Fire Fighting Measures
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
2、Handling and Storage
Handling: Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage: Store in a cool, dry place. Store in a tightly closed container. Corrosives area.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View