-
Name
5,6-Dimethoxy-1-indanone
- EINECS 218-287-8
- CAS No. 2107-69-9
- Article Data54
- CAS DataBase
- Density 1.179 g/cm3
- Solubility
- Melting Point 118-120 °C
- Formula C11H12O3
- Boiling Point 339.7 °C at 760 mmHg
- Molecular Weight 192.214
- Flash Point 150.4 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 22-24/25-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 5,6-Dimethoxyindan-1-one;5,6-dimethoxy-2,3-dihydroinden-1-one;2,3-Dihydro-5,6-dimethoxy-1H-inden-1-one;1H-Inden-1-one,2,3-dihydro-5,6-dimethoxy-;5,6-Dimethoxy Indanone;5,6-dimethoxy-1-indelene-1-one;
- PSA 35.53000
- LogP 1.83270
Synthetic route

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In dichloromethane at 80℃; for 1h; Friedel-Crafts Acylation; High pressure; Inert atmosphere; Green chemistry; | 100% |
| With trifluorormethanesulfonic acid In dichloromethane at 0 - 80℃; | 100% |
| With trifluorormethanesulfonic acid In dichloromethane at 0 - 80℃; for 1.5h; Sealed tube; | 96% |
-
-
52095-39-3
2-ethenyl-4,5-dimethoxybenzaldehyde

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; 6-diphenylphosphinomethyl-3-methyl-2-aminopyridine In toluene at 150℃; for 1h; Inert atmosphere; | 98% |
| With acetic acid; L-proline at 120℃; for 24h; Catalytic behavior; Reagent/catalyst; Temperature; Green chemistry; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-hydroxy-5-methoxyindan-1-one With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran at 20℃; for 1h; | 90.1% |

| Conditions | Yield |
|---|---|
| With pyridine; palladium diacetate; tetrabutyl-ammonium chloride In N,N-dimethyl-formamide at 100℃; under 760 Torr; for 24h; | 82% |
-
-
51842-87-6
3-(3,4-dimethoxy-phenyl)-propionyl chloride

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With rhenium(I) pentacarbonyl bromide In 1,2-dichloro-ethane for 2h; Heating; | 80% |
| With aluminium trichloride In dichloromethane at 20℃; for 3h; | 40.2 g |
| With aluminum (III) chloride In dichloromethane at 20℃; for 2h; |

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With silica gel; pyridinium chlorochromate In dichloromethane at 25℃; for 6h; | 72% |
-
-
64803-82-3
dimethyl 2-((3-(3,4-dimethoxyphenyl)propanoyl)oxy)succinate

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0 - 20℃; Friedel-Crafts Acylation; Inert atmosphere; regioselective reaction; | 70% |

| Conditions | Yield |
|---|---|
| With pyridine; tetrabutyl-ammonium chloride; tri tert-butylphosphoniumtetrafluoroborate; palladium diacetate In N,N-dimethyl-formamide at 130℃; for 0.333333h; microwave irradiation; | 67% |
-
-
2107-70-2
3,4-methoxycinnamic acid

-
A
-
2107-69-9
5,6-dimethoxy-1-indanone

-
B
-
57441-74-4
6,7-dimethoxy-2,3-dihydro-1H-inden-1-one

| Conditions | Yield |
|---|---|
| With terbium(III) trifluoromethanesulfonate In chlorobenzene at 250℃; for 1h; Friedel-Crafts reaction; | A 65% B 5% |
-
-
154317-78-9
5-(3,4-dimethoxybenzyl)-2,2-dimethyl-[1,3]dioxane-4,6-dione

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) In nitromethane at 100℃; for 0.75h; Friedel-Crafts acylation; | 59% |

| Conditions | Yield |
|---|---|
| With trifluoromethanesulfonic acid anhydride In 1,2-dichloro-ethane for 6h; Heating; | 42% |
| With trifluoromethylsulfonic anhydride; potassium carbonate 1.) dichloroethane, reflux, 6 h, 2.) dichloroethane, ether, 1 h; Yield given. Multistep reaction; |
-
-
33731-40-7
1-(3,4-dimethoxyphenyl)prop-2-en-1-one

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane for 5h; Heating; | 3.4% |
-
-
56661-87-1
6,7-dimethoxy-1-oxo-1H-isothiochromene-3-carboxylic acid

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With nickel |
-
-
62956-63-2
5,6-dimethoxy-3-oxo-indan-1-carboxylic acid

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With quinoline; copper at 230℃; |
-
-
40243-67-2
5,6-dimethoxy-1H-indene

-
A
-
2107-69-9
5,6-dimethoxy-1-indanone

-
B
-
30117-82-9
5,6-dimethoxy-1H-inden-2(3H)-one

| Conditions | Yield |
|---|---|
| With diborane In tetrahydrofuran a) 0 deg C, 15 min, b) 20 deg C, 45 min; | |
| With diborane In tetrahydrofuran a) 0 deg C, 15 min, b) 20 deg C, 45 min; Title compound not separated from byproducts; |
-
-
64-17-5
ethanol

-
-
56661-87-1
6,7-dimethoxy-1-oxo-1H-isothiochromene-3-carboxylic acid

-
-
2107-69-9
5,6-dimethoxy-1-indanone


| Conditions | Yield |
|---|---|
| With alkaline solution |

-
-
10026-13-8, 874483-75-7
phosphorus pentachloride

-
-
2107-70-2
3,4-methoxycinnamic acid

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| Behandlung des entstandenen Chlorids mit AlCl3; |

-
-
7664-93-9
sulfuric acid

-
-
106648-24-2
5,6-dimethoxy-1-oxo-2,3-dihydro-1H-indene-2-carbonitrile

-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: oxalyl chloride; DMF / CH2Cl2 2: 40.2 g / AlCl3 / CH2Cl2 / 3 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: piperidinium acetate / ethanol / 0.5 h 1.2: 77 percent / NaBH3CN / ethanol / 0 - 20 °C 2.1: 59 percent / Sc(OTf)3 / nitromethane / 0.75 h / 100 °C View Scheme |
-
-
61203-53-0
2-iodo-4,5-dimethoxybenzaldehyde

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: n-BuLi / tetrahydrofuran / 0.5 h / 0 °C 1.2: 90 percent / tetrahydrofuran 2.1: 82 percent / Pd(OAc)2; pyridine; n-Bu4NCl / dimethylformamide / 24 h / 100 °C / 760 Torr View Scheme |
-
-
33884-52-5
5,6-dimethoxy-2,3-dihydro-1H-inden-1-ol

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethyl sulfoxide / 6 h / 180 °C 2: diborane / tetrahydrofuran / a) 0 deg C, 15 min, b) 20 deg C, 45 min View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium amalgam; alkali 2: benzene; P2O5 View Scheme | |
| Multi-step reaction with 2 steps 1: H2 / Raney-Ni / ethanol / 100 °C / 66195.7 Torr 2: H3PO4, P2O5 View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 14 h / 60 °C / 114008 Torr 2: phosphorus pentoxide; phosphoric acid / 6 h / 120 °C View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / ethanol / 40 °C 2: trifluorormethanesulfonic acid / dichloromethane / 1 h / 80 °C View Scheme |
-
-
14737-89-4
3,4-dimethoxy-trans-cinnamic acid

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: platinum; acetic acid / Hydrogenation 2: HF View Scheme |
-
-
20583-78-2
3,4-dimethoxy-cinnamic acid ethyl ester

-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium hydroxide; methanol 2: sodium amalgam; alkali 3: benzene; P2O5 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) Py, (ii) aq. HCl 2: H2 / Raney-Ni / ethanol / 100 °C / 66195.7 Torr 3: H3PO4, P2O5 View Scheme | |
| Multi-step reaction with 2 steps 1: triethylammonium formate / N,N-dimethyl-formamide / 6 h / 20 °C 2: polyphosphoric acid / 2 h / 20 - 100 °C View Scheme | |
| Multi-step reaction with 3 steps 1: pyridine / 16 h / 80 °C 2: palladium 10% on activated carbon; hydrogen / ethyl acetate / 14 h / 60 °C / 114008 Torr 3: phosphorus pentoxide; phosphoric acid / 6 h / 120 °C View Scheme |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
616-38-6
carbonic acid dimethyl ester

-
-
119035-03-9
5,6-dimethoxy-2-methoxycarbonylindan-1-one

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil Reflux; Inert atmosphere; | 100% |
| With sodium hydride In paraffin oil at 90℃; | 92% |
| With sodium hydride In paraffin oil at 90℃; Inert atmosphere; | 92% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
22065-85-6
1-benzyl-4-formylpiperidine

-
-
120014-07-5
1-benzyl-4-<(5,6-dimethoxy-1-oxoindan-2-ylidenyl)methyl>piperidine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In water; toluene at 20℃; Product distribution / selectivity; Inert atmosphere; Reflux; | 100% |
| With sodium methylate In methanol; ethanol at 79℃; for 1.58333h; Product distribution / selectivity; Heating / reflux; | 93.4% |
| With sodium methylate In methanol at 66℃; for 1h; Product distribution / selectivity; Heating / reflux; | 93.9% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
124702-80-3
5,6-dihydroxy-indan-1-one

| Conditions | Yield |
|---|---|
| With boron tribromide In chloroform at -78℃; for 4h; | 100% |
| With boron tribromide In dichloromethane | 98% |
| With boron tribromide In dichloromethane at -78 - 20℃; for 1h; | 98% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; acetic acid In isopropyl alcohol at 90℃; under 1875.19 Torr; for 1.08333h; Microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 0.0125h; Irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In toluene at -10 - 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With bromine In ethyl acetate at 10 - 20℃; for 0.5h; | 98% |
| With bromine In methanol at 20℃; for 0.5h; | 84% |
| With copper(ll) bromide In ethyl acetate Reflux; | 81% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
17630-76-1
5-chloroindole 2,3-dione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetic acid at 100℃; for 1.25h; | 98% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
73568-29-3
2-chloro-6-methoxyquinolin-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 12h; | 98% |
-
-
872-85-5
pyridine-4-carbaldehyde

-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
877606-65-0
(E)-5, 6-dimethoxy-2-(pyridin-4-ylmethylene)-2, 3-dihydro-1H-inden-1-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 6h; Reflux; | 98% |
| With toluene-4-sulfonic acid In toluene for 6h; Heating; | 95.6% |
| With toluene-4-sulfonic acid In toluene for 6h; Reflux; | 90% |
-
-
872-85-5
pyridine-4-carbaldehyde

-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
4803-74-1
5,6-dimethoxy-2-[(pyridin-4-yl)methylene]-1-indanone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 25 - 30℃; for 3h; Product distribution / selectivity; | 98% |
| Stage #1: pyridine-4-carbaldehyde; 5,6-dimethoxy-1-indanone With toluene-4-sulfonic acid In toluene for 6h; Heating / reflux; Stage #2: With sodium carbonate In water for 0.5 - 1h; | 95.8% |
| With potassium iodide; calcium chloride In acetone at 40 - 50℃; for 4h; Reagent/catalyst; Time; | 92.2% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
105-58-8
Diethyl carbonate

-
-
53295-44-6
5,6-(dimethoxy)-2-(ethoxycarbonyl)-indan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: Diethyl carbonate With sodium hydride In tetrahydrofuran; oil for 1.5h; Heating / reflux; Stage #2: 5,6-dimethoxy-1-indanone In tetrahydrofuran; oil for 3h; Heating / reflux; | 98% |
| Stage #1: Diethyl carbonate With sodium hydride In tetrahydrofuran for 1.5h; Heating / reflux; Stage #2: 5,6-dimethoxy-1-indanone In tetrahydrofuran for 3h; Heating / reflux; | 98% |
| With sodium hydride In toluene; mineral oil for 6h; Reflux; | 85% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
100-51-6
benzyl alcohol

-
-
74402-02-1
2-benzyl-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one

| Conditions | Yield |
|---|---|
| With potassium phosphate tribasic trihydrate; C39H32Cl2N5PRu In tert-Amyl alcohol at 120℃; for 4h; Inert atmosphere; Schlenk technique; | 98% |
| With trifuran-2-yl-phosphane; C48H34F6N4O4Pd2; lithium hydroxide In neat (no solvent) at 100℃; for 24h; Inert atmosphere; Schlenk technique; Sealed tube; | 92% |

| Conditions | Yield |
|---|---|
| With potassium phosphate tribasic trihydrate; C39H32Cl2N5PRu In tert-Amyl alcohol at 120℃; for 4h; Inert atmosphere; Schlenk technique; | 98% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
917-54-4
methyllithium

-
-
127600-31-1
5,6-dimethoxy-3-methyl-1H-indene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether for 16h; Ambient temperature; -55 deg C to room temperature; | 97% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
537690-41-8
3-bromo-5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane for 2.5h; Heating; | 97% |
| With N-Bromosuccinimide; dibenzoyl peroxide In tetrachloromethane for 4h; Reagent/catalyst; Solvent; Reflux; | 80.4% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
113211-42-0
2-butenyloxyamine hydrochloride

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol for 2h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; n-Butyl nitrite In methanol at 40℃; for 0.5h; | 95% |
| With hydrogenchloride; n-Butyl nitrite In methanol; water at 40℃; for 2.5h; | 93% |
| With hydrogenchloride; n-Butyl nitrite In methanol at 40℃; for 0.5h; | 92% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With bromine In acetic acid at 20℃; for 2h; | 95% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 20℃; for 48h; Claisen-Schmidt Condensation; | 95% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene Reflux; Dean-Stark; Inert atmosphere; | 95% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
59078-28-3
5,6-dimethoxyindane

| Conditions | Yield |
|---|---|
| With hydrogenchloride; mercury; zinc In water; toluene for 6h; Heating; | 94% |
| With amalgamated zinc | 71% |
| With hydrogenchloride; mercury; zinc |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetic acid at 100℃; for 1.25h; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetic acid at 105℃; for 16h; | 94% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
68236-25-9
2-chloro-5,6,7-trimethoxyquinoline-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 12h; | 94% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water at 50℃; for 72h; | 93.8% |
-
-
2107-69-9
5,6-dimethoxy-1-indanone

-
-
91247-06-2
5,6-dimethoxy-1-aminoindane

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In ethanol at 100℃; under 3750.38 - 33753.4 Torr; Pressure; Autoclave; | 93.3% |
| Multi-step reaction with 2 steps 1: hydroxylamine hydrochloride, NaOH, H2O / ethanol / 0.33 h / Heating 2: Raney nickel, NaOH, H2O / ethanol / 1 h View Scheme |
5,6-Dimethoxy-1-indanone Chemical Properties
Molecular Structure of 5,6-Dimethoxyindan-1-one (CAS NO. 2107-69-9):
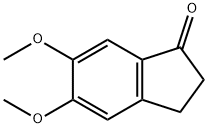
IUPAC Name: 5,6-Dimethoxy-2,3-dihydroinden-1-one
Molecular Formula: C11H12O3
Molecular Weight: 192.211180 g/mol
Index of Refraction: 1.549
Molar Refractivity: 51.91 cm3
Molar Volume: 163 cm3
Polarizability: 20.58 × 10-24 cm3
Surface Tension: 41.9 dyne/cm
Density: 1.179 g/cm3
Flash Point: 150.4 °C
Enthalpy of Vaporization: 58.32 kJ/mol
Boiling Point: 339.7 °C at 760 mmHg
Vapour Pressure: 9.02E-05 mmHg at 25 °C
Melting Point: 118-120 °C
EINECS: 218-287-8
Product Categories: Indane/Indanone and Derivatives;Indanone & Indene;API intermediates;Aromatics Compounds;Intermediates of Donepezil;Aromatics;C11 to C12;Carbonyl Compounds;Ketones
5,6-Dimethoxy-1-indanone Safety Profile
Safety Information of 5,6-Dimethoxyindan-1-one (CAS NO. 2107-69-9):
Hazard Codes: Xn,Xi 
Risk Statements: 20/21/22-36/37/38
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed
R36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 22-24/25-36-26
S22:Do not breathe dust
S24/25:Avoid contact with skin and eyes
S36:Wear suitable protective clothing
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
WGK Germany: 3
5,6-Dimethoxy-1-indanone Specification
5,6-Dimethoxyindan-1-one with cas registry number of 2107-69-9 is white to light yellow crystal powder, also called NSC 401450 ; 1H-Inden-1-one, 2,3-dihydro-5,6-dimethoxy- .
Related Products
- 5-0185 (Beilstein Handbook Reference) 6850-22-2
- 5,10,15,20-Tetra(4-methylphenyl)-21H,23H-porphine
- 5,10,15,20-Tetra(4-pyridyl)porphyrin
- 5,10,15,20-Tetrakis(2,4,6-trimethylphenyl)-21H,23H-porphine
- 5,10,15,20-Tetrakis(4-hydroxyphenyl)porphyrin
- 5,10,15,20-Tetrakis(4-methoxyphenyl)-21H,23H-porphine
- 5,10,15,20-Tetrakis(N-methyl-4-pyridyl)porphine tetratosylate
- 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin
- 5,10,15,20-Tetraphenyl-21H,23H-porphine cobalt(II)
- 5,10,15,20-Tetraphenyl-21H,23H-porphine iron(III) chloride
- 2107-70-2
- 21077-27-0
- 2107-76-8
- 2107-78-0
- 21078-81-9
- 21080-66-0
- 21080-92-2
- 2108-20-5
- 210821-63-9
- 21082-26-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View