-
Name
5-Methoxyindole
- EINECS 213-745-3
- CAS No. 1006-94-6
- Article Data121
- CAS DataBase
- Density 1.169 g/cm3
- Solubility insoluble in water
- Melting Point 52-55 °C(lit.)
- Formula C9H9NO
- Boiling Point 311.9 °C at 760 mmHg
- Molecular Weight 147.177
- Flash Point 109.2 °C
- Transport Information 50kgs
- Appearance white to light brownish crystalline powder
- Safety 26-36-24/25-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Indole, 5-methoxy-;1H-Indole,5-methoxy-;1/C9H9NO/c1-11-8-2-3-9-7(6-8)4-5-10-9/h2-6,10H,1H;Methoxy-5 indole [French];Femedol;5-methoxy indole;5-methyloxyl indole;5-Methoxylindole;5-methoxy-indole;5-Methyoxy Indole;5-Methoxyindole 98%;Methoxy-5 indole [French];1H-Indole, 5-methoxy-;Methoxy-5 indole;5-Methoxy-1H-indole;Indol-5-yl methyl ether;1H-Indole, 5-methoxy- (9CI);
- PSA 25.02000
- LogP 2.17650
Synthetic route
-
-
139717-71-8
5-methoxy-N-(4-toluenesulfonyl)-1H-indole

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl acetamide at 60℃; for 5h; Inert atmosphere; | 90% |
| With formic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 24h; Inert atmosphere; Sealed tube; Irradiation; | 85% |
| With caesium carbonate In tetrahydrofuran; methanol at 64℃; for 2.5h; | |
| With cetyltrimethylammonim bromide; potassium hydroxide In tetrahydrofuran; water for 120h; Reflux; Green chemistry; |
-
-
10601-19-1
5-methoxyindole-3-carboxaldehyde

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate In ethyl acetate at 150℃; under 12929 Torr; for 0.833333h; Microwave irradiation; Molecular sieve; | 90% |
| With perchloric acid adsorbed on silica gel; anthranilic acid amide In acetonitrile at 80℃; for 6h; | 74% |
-
-
10242-01-0
5-Methoxyindole-3-carboxylic acid

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 140℃; Schlenk technique; | 99% |
-
-
32989-62-1
4-methoxy-trans-2-[β-(dimethylamino)vinyl]-nitrobenzene

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In benzene at 25℃; under 2280 Torr; for 27h; | 68% |
| palladium In benzene |
-
-
845619-77-4
1-(5-methoxy-1H-indol-1-yl)-2,2-dimethylpropan-1-one

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With water; 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran for 18h; Reflux; | 99% |
| With lithium diisopropyl amide In tetrahydrofuran; hexane at 40 - 45℃; for 2h; | 92% |
-
-
126759-31-7
2-vinyl-4-methoxynitrobenzene

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With carbon monoxide; triphenylphosphine; palladium diacetate In acetonitrile under 3040 Torr; for 19h; Heating; | 63% |
| Multi-step reaction with 2 steps 1: 50 percent / O2 (1 atm) / Na2PdCl4 / methanol / 24 h / Ambient temperature 2: 63 percent / Fe, acetic acid, 10percent HCl / ethanol / Temp. 70 - 75 deg C, 1 h. Temp. 85 deg C, 2 h View Scheme | |
| Multi-step reaction with 2 steps 1: 85 percent / oxygen / palladium(II) chloride, copper(I) chloride / 1,2-dimethoxy-ethane / 24 h / 50 - 60 °C 2: 1.) H2, 2.) aq. HCl / 10percent rhodium-carbon / 1.) ethanol, room temperature, 1 atmosphere, 3 h, 2.) room temperature, 3 h View Scheme |
-
-
20357-24-8
5-methoxy-2-nitro-benzaldehyde

-
-
86302-43-4
(tert-Butoxycarbonylmethylene)triphenylphosphorane

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With triphenylphosphine In diphenylether at 260℃; for 1h; | 51% |

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With hydrogen In N,N-dimethyl-formamide at 330 - 340℃; under 15001.5 Torr; for 10h; Pressure; Temperature; |
-
-
89302-15-8
2-(5-methoxy-2-nitrophenyl)acetonitrile

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 20℃; under 760.051 Torr; for 12h; | 88% |
| With hydrogen; acetic acid; 10% palladium on active carbon In ethanol under 2280 Torr; for 2h; Ambient temperature; | 83% |
| With hydrogen at 20℃; under 760.051 Torr; for 24h; Schlenk technique; | 68% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide In N,N-dimethyl-formamide for 6h; Reflux; | 98% |
-
-
107127-61-7
(E)-5-methoxy-2-nitro-β-morpholinestyrene

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In benzene at 50℃; under 4560 Torr; for 8h; | 72% |
-
-
99275-47-5
1-(tert-butyloxycarbonyl)-5-methoxyindole

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| In various solvent(s) at 150℃; for 0.25h; microwave irradiation; | 98% |
| With 1,1,1,3',3',3'-hexafluoro-propanol at 150℃; for 0.25h; Product distribution / selectivity; Microwave irradiation; | 98% |
| With 1,1,1,3',3',3'-hexafluoro-propanol at 150℃; for 0.25h; Product distribution / selectivity; Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With tin(ll) chloride; dihydridotetrakis(triphenylphosphine)ruthenium In 1,4-dioxane; water at 180℃; for 20h; | 80% |
| With triphenylphosphine; tin(ll) chloride; ruthenium trichloride In 1,4-dioxane; water at 180℃; for 20h; Cyclization; | 43% |
-
-
21857-45-4
5-methoxyindoline

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With H8O20P8Pt2(4-)*4C34H72N(1+) In methanol Inert atmosphere; Irradiation; | 99% |
| With 6C44H32N6O4Ru(2+)*12Hf(2+)*8O(2-)*14HO(1-)*6C16H22ClCoN5O6(1-) In 2,2,2-trifluoroethanol; acetonitrile at 20℃; for 12h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Irradiation; | 96% |
| With C21H21ClIrNO2 In tetrahydrofuran; 2,2,2-trifluoroethanol for 20h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| In quinoline for 0.2h; microwave; | 100% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In sulfolane at 300℃; for 0.333333h; Inert atmosphere; | 87% |
| With quinoline; copper Heating; | 77% |
| With quinoline; copper oxide-chromium oxide at 200 - 210℃; |

| Conditions | Yield |
|---|---|
| With tin(ll) chloride; ruthenium trichloride; triphenylphosphine In 1,4-dioxane at 180℃; for 20h; | 33% |

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With silica gel In 5,5-dimethyl-1,3-cyclohexadiene at 170℃; Retro Diels-Alder reaction; Inert atmosphere; | 97% |
-
-
261762-11-2
5-methoxy-1-[(2-nitrophenyl)methyl]-1H-indole

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With hydrazine In 1,4-dioxane; water for 0.333333h; Inert atmosphere; UV-irradiation; | 30% |

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 68% B 71% |

-
-
21857-45-4
5-methoxyindoline

-
-
99-12-7
5-nitro-m-xylene

-
A
-
1006-94-6
5-methoxylindole

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 62% B 61% |

-
-
124043-85-2
2-(2-amino-5-methoxyphenyl)ethyl alcohol

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| tris(triphenylphosphine)ruthenium(II) chloride In toluene for 6h; Heating; | 94% |
| tris(triphenylphosphine)ruthenium(II) chloride In toluene for 6h; Rate constant; Heating; | 94% |
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; potassium carbonate In toluene at 111℃; for 20h; | 68% |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2.89 g / 7 h / 148 - 150 °C 2: 72 percent / H2 / Pd/C / benzene / 8 h / 50 °C / 4560 Torr View Scheme | |
| Multi-step reaction with 5 steps 1: N-bromosuccinimide (NBS), azoisobutyronitrile (AIBN) / CCl4 / 3 h / Heating 2: toluene / 1.) 6 h, RT; 2.) 3 h, 100 deg C 3: 74 percent / 48percent HBr, Zn / ethanol / 2 h / Heating 4: 87 percent / 2.5 h / Heating 5: 82 percent / t-BuOK / toluene / 0.25 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 4 h / 110 °C 2: TiCl3, 4M NH4OAc / acetone View Scheme |
-
-
119730-01-7
2-chloro-5-methoxy-1H-indole

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate Ambient temperature; | 93% |
-
-
21857-45-4
5-methoxyindoline

-
-
81-20-9
2,6-dimethylnitrobenzene

-
A
-
1006-94-6
5-methoxylindole

-
B
-
87-62-7
2,6-dimethylaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 40% B 41% |

-
-
61293-32-1
1-[(E)-2-(5-methoxy-2-nitrophenyl)vinyl]pyrrolidine

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate; pyrographite; hydrazine hydrate In ethanol at 75℃; for 5h; | 91% |
-
-
88131-62-8
1-(Methylsulfonyl)-5-methoxyindole

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 18h; Heating; | 91% |

| Conditions | Yield |
|---|---|
| With phosphomolybdic acid In chloroform at 20 - 60℃; Fischer indole synthesis; | 88% |

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; sodium methylate; copper(I) bromide at 120℃; for 10h; Time; Reagent/catalyst; Temperature; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) LiAlH4 / tetrahydrofuran / 0 °C / 1.) 15 min, 2.) 45 min 2: MnO2 / CH2Cl2 / 1 h / 25 °C 3: 95 percent / Rh(dppp)2Cl / 18 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: aq.-ethanolic KOH 2: quinoline; copper oxide-chromium oxide / 200 - 210 °C View Scheme | |
| Stage #1: ethyl 5-methoxy-1H-indole-2-carboxylate With sodium hydroxide In ethanol at 40℃; Stage #2: With quinoline; copper at 250℃; for 0.416667h; Microwave irradiation; |
-
-
194869-21-1
(4-Methoxy-2-trimethylsilanylethynyl-phenyl)-carbamic acid tert-butyl ester

-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol for 17h; Heating; | 67% |
-
-
1006-94-6
5-methoxylindole

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
10601-19-1
5-methoxyindole-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 0 - 20℃; | 100% |
| With sodium hydroxide; trichlorophosphate Vilsmeier-Haack reaction; | 98% |
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 0℃; for 0.333333h; Stage #2: 5-methoxylindole at 0 - 20℃; for 1.5h; | 98% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Ambient temperature; | 100% |
| In 1,4-dioxane at 20℃; for 0.5h; |
-
-
1006-94-6
5-methoxylindole

-
-
21857-45-4
5-methoxyindoline

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid at 0 - 20℃; for 2h; | 100% |
| With platinum/carbon xerogel catalyst; hydrogen; toluene-4-sulfonic acid In water at 25℃; under 22502.3 Torr; for 3h; regioselective reaction; | 96% |
| With C26H29ClIrNO3; hydrogen In 2,2,2-trifluoroethanol at 20℃; under 760.051 Torr; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 95 - 100℃; for 2h; | 100% |
-
-
1006-94-6
5-methoxylindole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
99275-47-5
1-(tert-butyloxycarbonyl)-5-methoxyindole

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran at 20℃; for 16h; Acylation; | 100% |
| With dmap; triethylamine In 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran at 20℃; for 19h; | 99% |
| dmap In acetonitrile at 20℃; | 97% |
-
-
1006-94-6
5-methoxylindole

-
-
22445-41-6
3,5-dimethylphenyl iodide

-
-
360045-08-5
2,3-dihydro-5-methoxy-1-(3,5-dimethylphenyl)-1H-indole

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; (S,S)-1,2-diaminocyclohexane In 1,4-dioxane; dodecane at 110℃; for 24h; | 100% |
-
-
1006-94-6
5-methoxylindole

-
-
22445-41-6
3,5-dimethylphenyl iodide

-
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

-
-
360045-08-5
2,3-dihydro-5-methoxy-1-(3,5-dimethylphenyl)-1H-indole

| Conditions | Yield |
|---|---|
| With potassium phosphate; CuI In 1,4-dioxane | 100% |

-
-
1006-94-6
5-methoxylindole

-
-
96-32-2
bromoacetic acid methyl ester

-
-
885524-56-1
methyl 2-(5-methoxy-1H-indol-1-yl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: 5-methoxylindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.75h; Stage #2: bromoacetic acid methyl ester In N,N-dimethyl-formamide; mineral oil at 20℃; for 15h; | 100% |
| Stage #1: 5-methoxylindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.75h; Stage #2: bromoacetic acid methyl ester In N,N-dimethyl-formamide; mineral oil at 20℃; for 15h; | 100% |
-
-
869789-68-4
(E)-3-(4-methoxyphenyl)-1-(1-methyl-1H-imidazol-2-yl)prop-2-en-1-one

-
-
1006-94-6
5-methoxylindole

-
-
1242049-84-8
3-(5-methoxy-1H-indol-3-yl)-3-(4-methoxyphenyl)-1-(1-methyl-1H-imidazol-2-yl)propan-1-one

| Conditions | Yield |
|---|---|
| With 4,4'-dimethyl-2,2'-bipyridines; copper(II) nitrate trihydrate In acetonitrile at 20℃; for 72h; Friedel-Crafts Alkylation; Inert atmosphere; | 100% |
| With 4,4'-dimethyl-2,2'-bipyridines; copper(II) nitrate trihydrate In acetonitrile at 20℃; for 72h; Friedel-Crafts Alkylation; Inert atmosphere; | 100% |
| With copper(II) nitrate trihydrate In aq. buffer at 20℃; for 72h; pH=6.5; Friedel-Crafts Alkylation; | 85% |
| With copper(II) nitrate trihydrate In aq. buffer at 20℃; for 72h; pH=6.5; Reagent/catalyst; Friedel-Crafts Alkylation; enantioselective reaction; | 85% |
-
-
869789-69-5
(E)-3-(4-chlorophenyl)-1-(1-methyl-1H-imidazol-2-yl)prop-2-en-1-one

-
-
1006-94-6
5-methoxylindole

-
-
1242049-82-6
3-(4-chlorophenyl)-3-(5-methoxy-1H-indol-3-yl)-1-(1-methyl-1H-imidazol-2-yl)propan-1-one

| Conditions | Yield |
|---|---|
| With 4,4'-dimethyl-2,2'-bipyridines; copper(II) nitrate trihydrate In acetonitrile at 20℃; for 72h; Friedel-Crafts Alkylation; Inert atmosphere; | 100% |
| With 4,4'-dimethyl-2,2'-bipyridines; copper(II) nitrate trihydrate In acetonitrile at 20℃; for 72h; Catalytic behavior; Reagent/catalyst; Friedel-Crafts Alkylation; Inert atmosphere; | 100% |
| With 4,4'-dimethyl-2,2'-bipyridines; copper(II) nitrate trihydrate In acetonitrile at 20℃; for 72h; Friedel-Crafts Alkylation; Inert atmosphere; | 100% |
-
-
1006-94-6
5-methoxylindole

| Conditions | Yield |
|---|---|
| With iodine In methanol at 20℃; for 24h; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With iodine; triphenylphosphine In ethanol at 70℃; for 24h; regioselective reaction; | 100% |
-
-
1006-94-6
5-methoxylindole

-
-
645-96-5
Benzeneselenol

-
-
1599461-87-6
5-methoxy-3-(phenylselanyl)-1H-indole

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 27℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-methoxylindole With sodium hydride In acetonitrile at 0℃; for 0.5h; Stage #2: methyl iodide In acetonitrile at 0 - 20℃; for 16h; | 99% |
| Stage #1: 5-methoxylindole With Tris(3,6-dioxaheptyl)amine; potassium tert-butylate In benzene at 20℃; for 1h; Stage #2: methyl iodide In benzene at 20℃; for 0.5h; | 97% |
| With sodium hydroxide In dimethyl sulfoxide at 20℃; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: nitrostyrene With (S)-10,10'-bis[(S)-4-isopropyl-4,5-dihydrooxazol-2-yl]-9,9'-biphenanthrene; zinc(II) trifluoroacetate In diethyl ether at 20℃; for 0.25h; Inert atmosphere; Stage #2: 5-methoxylindole In diethyl ether at 20℃; Friedel-Crafts alkylation; Inert atmosphere; | 99% |
| With N,N'-bis(3,5-bis(trifluoromethyl)benzyl)-2-nitroethene-1,1-diamine In toluene at 20℃; for 24h; Michael Addition; | 99% |
| With [(1S)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]methanesulfonic acid In water at 20℃; for 8h; Friedel-Crafts Alkylation; Green chemistry; | 94% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 3h; Electrochemical reaction; Inert atmosphere; | 99% |
| With Oxone In methanol at 20℃; for 0.35h; | 98% |
| With oxygen In tetrahydrofuran at 25℃; under 750.075 Torr; for 7h; Irradiation; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-methoxylindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h; Stage #2: benzyl bromide In N,N-dimethyl-formamide; mineral oil at 20℃; | 99% |
| Stage #1: 5-methoxylindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; Inert atmosphere; Stage #2: benzyl bromide In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; Inert atmosphere; | 97% |
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; Inert atmosphere; | 94% |
-
-
1006-94-6
5-methoxylindole

-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

| Conditions | Yield |
|---|---|
| With (SIr,RC)-[(η5-C5Me5)Ir{(R)-propane-1,2-diylbis(diphenylphosphane)}(H2O)][SbF6]2 In dichloromethane for 0.333333h; Friedel-Crafts Alkylation; Inert atmosphere; Schlenk technique; Optical yield = 71 %ee; | 99% |
| With K-10 montmorillonite In toluene at 60℃; for 0.0833333h; Friedel-Crafts hydroxyalkylation; | 97% |
| In 1,1,1,3,3-pentafluorobutane at 20℃; for 0.5h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With norborn-2-ene; dichloro bis(acetonitrile) palladium(II); potassium carbonate In N,N-dimethyl acetamide; water at 70℃; regioselective reaction; | 99% |
| With potassium acetate; palladium diacetate; bis-diphenylphosphinomethane In water at 110℃; for 24h; regioselective reaction; | 79% |
| With bis(di-tert-butyl(4-dimethylaminophenyl)phosphine)dichloropalladium(II); tetrachlorophthalic anhydride; sodium hydroxide In ethanol; water at 80℃; for 24h; regioselective reaction; | 73% |
| With cesium acetate; palladium diacetate In N,N-dimethyl acetamide at 125℃; for 24h; | 64% |
| With norborn-2-ene; palladium diacetate; potassium carbonate In N,N-dimethyl acetamide; water at 70℃; |

| Conditions | Yield |
|---|---|
| With di(naphthalen-1-yl)silanediol In dichloromethane at 23℃; for 48h; Inert atmosphere; | 99% |
| With 2,6-bis(2,2-dimethylpropionylamino)benzoic acid In chloroform at 40℃; for 24h; Friedel-Crafts Alkylation; | 99% |
| With 1,1,1,3',3',3'-hexafluoro-propanol at 20℃; for 2h; Friedel-Crafts Alkylation; | 96% |
-
-
1006-94-6
5-methoxylindole

-
-
431-46-9
2,2,2-trifluoro-1-methoxy-ethanol

-
-
24313-88-0
3,4,5-Trimethoxyaniline

-
-
1132829-45-8
N-((R)-2,2,2-trifluoro-1-(5-methoxy-1H-indol-3-yl)ethyl)-3,4,5-trimethoxybenzenamine

| Conditions | Yield |
|---|---|
| With (S)-3,3'-bis(2,4,6-tri-iso-propylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In dichloromethane at 20℃; for 24h; Friedel Crafts aminoalkylation; Molecular sieve; optical yield given as %ee; enantioselective reaction; | 99% |

-
-
1006-94-6
5-methoxylindole

-
-
15725-33-4
diethyl m-methylbenzylidenemalonate

| Conditions | Yield |
|---|---|
| Stage #1: 5-methoxylindole With C45H52N4O4; scandium tris(trifluoromethanesulfonate) In tert-butyl alcohol at 35℃; for 1h; Inert atmosphere; Stage #2: diethyl m-methylbenzylidenemalonate In diethyl ether at -20℃; for 118h; Friedel Crafts alkylation; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
1006-94-6
5-methoxylindole

-
-
434-45-7
1,1,1-trifluoroacetophenone

-
-
1150561-10-6
2,2,2-trifluoro-1-(5-methoxy-1H-indol-3-yl)-1-phenylethan-1-ol

| Conditions | Yield |
|---|---|
| With tetrabutyl phosphonium bromide; potassium carbonate In water at 20℃; for 12h; Reagent/catalyst; regioselective reaction; | 99% |
| With N,N,N′,N′-tetramethyl-N″-tert-butylguanidine; water at 20℃; for 16h; Friedel-Crafts type alkylation; | 98% |
| With TMG In dichloromethane at 20℃; | |
| With diphenyl hydrogen phosphate In dichloromethane at 0℃; for 20h; Friedel-Crafts Alkylation; Molecular sieve; Inert atmosphere; Sealed tube; |
-
-
1006-94-6
5-methoxylindole

-
-
434-45-7
1,1,1-trifluoroacetophenone

-
-
1160936-79-7
(R)-2,2,2-trifluoro-1-(5-methoxy-1H-indol-3-yl)-1-phenylethanol

| Conditions | Yield |
|---|---|
| With (S)-3,3'-bis(2,4,6-tri-iso-propylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In dichloromethane at 25℃; for 48h; optical yield given as %ee; enantioselective reaction; | 99% |
5-Methoxyindole Specification
The 5-Methoxyindole, with the CAS registry number 1006-94-6, is also known as 1H-Indole, 5-methoxy- (9CI). It belongs to the product categories of Blocks; Indoles Oxindoles; Heterocycles; Indoles and Derivatives; Indole Derivative; Pyrroles & Indoles; Indoline & Oxindole; Indole; Indoles; Heterocyclic Compounds; Indole Series; Indole Derivatives; Simple Indoles; Chiral Compound; Pyrroles & Indoles; Building Blocks; Heterocyclic Building Blocks. Its EINECS registry number is 213-745-3. This chemical's molecular formula is C9H9NO and molecular weight is 147.17. Its IUPAC name is called 5-methoxy-1H-indole. What's more, this chemical's classification code is Drug / Therapeutic Agent. 5-Methoxyindole is used as intermediate of pharmaceuticals.
Physical properties of 5-Methoxyindole: (1)ACD/LogP: 2.06; (2)ACD/LogD (pH 5.5): 2.06; (3)ACD/LogD (pH 7.4): 2.06; (4)ACD/BCF (pH 5.5): 21.57; (5)ACD/BCF (pH 7.4): 21.57; (6)ACD/KOC (pH 5.5): 313.65; (7)ACD/KOC (pH 7.4): 313.65; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Index of Refraction: 1.637; (12)Molar Refractivity: 45.2 cm3; (13)Molar Volume: 125.8 cm3; (14)Surface Tension: 45.7 dyne/cm; (15)Density: 1.169 g/cm3; (16)Flash Point: 109.2 °C; (17)Enthalpy of Vaporization: 53.08 kJ/mol; (18)Boiling Point: 311.9 °C at 760 mmHg; (19)Vapour Pressure: 0.00101 mmHg at 25°C.
Preparation of 5-Methoxyindole: this chemical can be prepared by 5-methoxy-indole-2-carboxylic acid. This reaction will need reagents quinoline and copper oxide-chromium oxide. The reaction temperature is 200 - 210 °C.

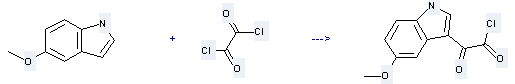
Uses of 5-Methoxyindole: it can be used to produce (5-methoxy-indol-3-yl)-oxoacetyl chloride. This reaction will need reagent diethyl ether.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: COC1=CC2=C(C=C1)NC=C2
(2)InChI: InChI=1S/C9H9NO/c1-11-8-2-3-9-7(6-8)4-5-10-9/h2-6,10H,1H3
(3)InChIKey: DWAQDRSOVMLGRQ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 370mg/kg (370mg/kg) | European Journal of Medicinal Chemistry--Chimie Therapeutique. Vol. 9, Pg. 453, 1974. |
Related Products
- 5-Methoxyindole
- 5-Methoxyindole-2-carboxylic acid
- 5-Methoxyindole-3-acetic acid
- 5-Methoxyindole-3-acetonitrile
- 5-Methoxyindole-3-carboxaldehyde
- 5-Methoxyindole-3-ethanol
- 100-69-6
- 1006-99-1
- 1007-01-8
- 1007-03-0
- 100704-10-7
- 1007-06-3
- 100707-39-9
- 1007089-84-0
- 100-70-9
- 10070-92-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View