-
Name
5-Nitro-1,10-phenanthroline
- EINECS 224-097-6
- CAS No. 4199-88-6
- Article Data40
- CAS DataBase
- Density 1.444 g/cm3
- Solubility insoluble
- Melting Point 202-204 ºC (lit.)
- Formula C12H7N3O2
- Boiling Point 444ºC at 760 mmHg
- Molecular Weight 225.206
- Flash Point 222.3 ºC
- Transport Information
- Appearance Pale yellow or brown solid
- Safety S26-36-24/25-22
- Risk Codes R36/37/38-40
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn Xi
Xi
- Synonyms 5-Nitro-1,10-phenanthroline;5-Nitro-o-phenanthroline;NSC 4263;
- PSA 71.60000
- LogP 3.21440
Synthetic route

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 160℃; for 3h; | 100% |
| With sulfuric acid; nitric acid at 160℃; | 99% |
| With sulfuric acid; nitric acid at 160℃; for 3h; | 99% |
-
-
630067-06-0
1,10-phenanthroline-5-carboxylic acid

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With nitronium tetrafluoborate; silver carbonate In N,N-dimethyl acetamide at 90℃; for 12h; Inert atmosphere; Schlenk technique; regioselective reaction; | 81% |
-
-
66-71-7
1,10-Phenanthroline

-
A
-
27318-90-7
1,10-phenanthroline-5,6-dione

-
B
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid for 1.5h; Heating; | A 20% B 18.7 g |

| Conditions | Yield |
|---|---|
| With sulfuric acid; orthoarsenic acid |
-
-
66-71-7
1,10-Phenanthroline

-
-
7664-93-9
sulfuric acid

-
-
7446-11-9
sulfur trioxide

-
-
7697-37-2
nitric acid

-
A
-
50890-67-0
4,5-Diazafluoren-9-one

-
B
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| at 170℃; |

-
-
66-71-7
1,10-Phenanthroline

-
-
7664-93-9
sulfuric acid

-
-
7446-11-9
sulfur trioxide

-
-
7697-37-2
nitric acid

-
A
-
27318-90-7
1,10-phenanthroline-5,6-dione

-
B
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| at 170℃; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: glycerol; H3aso4; aqueous H2SO4 2: H2SO4; concentrated HNO3 View Scheme |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid | 1.49 g (42%) |
-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
54258-41-2
1,10-phenanthroline-5-amine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 2280.15 Torr; for 1h; | 100% |
| With 10% Pd/C; hydrogen In methanol under 2280.15 Torr; for 1h; | 100% |
| With hydrazine hydrate; palladium 10% on activated carbon In ethanol for 4h; Reflux; | 98% |
-
-
34035-97-7
ammonium pertechnetate

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol 5-nitro-1,10-phenanthroline dissolved in MeOH by gentle heating, cooled to ambient temp., addn. of Tc-compd. with stirring, dropwise addn. of concd. HCl; cooled to -20°C, ppt. collected, washed with MeOH, dried in vac. overnight; elem. anal.; | 99% |
-
-
37366-09-9
dichloro(benzene)ruthenium(II) dimer

-
-
75-09-2
dichloromethane

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| In dichloromethane inert atm.; 2 equiv of phenanthroline was added to a suspn. of complex in CH2Cl2, the mixt. was stirred for 3 h at room temp.; evapd. to dryness, dissolved in water, filtered, evapd. to dryness; elem. anal.; | 99% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
861392-80-5
[(η6-hexamethylbenzene)Ru(η2-5-nitro-1,10-phenanthroline)Cl]Cl

| Conditions | Yield |
|---|---|
| In dichloromethane inert atm.; 2 equiv of phenanthroline was added to a suspn. of complex in CH2Cl2, the mixt. was stirred for 3 h at room temp.; evapd. to dryness, dissolved in water, filtered, evapd. to dryness; elem. anal.; | 99% |
-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
861392-79-2
[(η6-p-cymene)Ru(nitrophen)Cl]Cl

| Conditions | Yield |
|---|---|
| In dichloromethane inert atm.; 2 equiv of phenanthroline was added to a suspn. of complex in CH2Cl2, the mixt. was stirred for 3 h at room temp.; evapd. to dryness, dissolved in water, filtered, evapd. to dryness; elem. anal.; | 99% |
| In dichloromethane for 2h; Reflux; |
-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
93102-05-7
N-benzyl-N-(methoxymethyl)-N-[(trimethylsilyl)methyl]amine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 0 - 20℃; for 5h; Inert atmosphere; | 99% |

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
65060-51-7
(Pd(5-nitro-1,10-phenanthroline)(SCN)2)

| Conditions | Yield |
|---|---|
| In ethanol dropwise addn. of stoich. amt. of ligand to Pd-complex (60°C, pptn.); filtration, washing (H2O, EtOH, Et2O), drying (in air); elem. anal.; | 96% |

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| Stage #1: 5-Nitro-1,10-phenanthroline; C116H84Cl2Ir2N8 In methanol; dichloromethane for 6h; Reflux; Stage #2: ammonium hexafluorophosphate In methanol; dichloromethane for 0.25h; | 95% |

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
1360775-94-5
[{(η3-C3H4COOMe)Mo(CO)2(NCMe)(μ-Br)}2]

-
-
1394903-11-7
[(η3-C3H4COOMe)Mo(CO)2(5-NO2-phen)Br]

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.5h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| In acetonitrile dissolving of Cu(BF4)2 hydrate (1 equiv.) in CH3CN, addn. dropwise of soln. of NO2C12H7N2 (2 equiv.) in CH3CN; removal of solvent by rotary evapn.; | 93.01% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
1394903-08-2
[(η3-C3H5)Mo(CO)2(5-NO2-phen)Cl]

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.5h; Inert atmosphere; | 93% |

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With piperidinium 2-chloroterephthalate In methanol addn. of soln. of nitrophenanthroline to soln. of Mn salt and piperidinium chloroterephthalate, stirring (6 h, room temp.; pptn.); filtration, washing (EtOH, Et2O), drying (vac.); elem. anal.; | 92% |

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Inert atmosphere; | 92% |

-
-
25148-93-0
N,N'-bis<2-(dimethylamino)ethyl>oxamide

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With triethylamine In methanol to soln. of oxamide stirred in MeOH added soln. of Cu(ClO4)2*6H2O in MeOH and Et3N; soln. stirred for 30 min at room temp.; filtered; to filtrate added soln. of Mn(ClO4)2*6H2O in MeOH and 5-NO2-1,10-phenanthroline inMeOH under N2; mixt. stirred for 5 h; crystals filtered; washed (MeOH, H2O, Et2O); dried (P2O5, vac.); recrystd. (DMF/EtOH (1:1)); elem. anal.; | 90% |


-
-
20102-49-2
N,N'-bis(3-aminopropyl)oxamido copper(II)

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With ethyl orthoformate In ethanol elem. anal.; | 90% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| In water suspn. of bis(2,2'-bipyridine)RuCl2 and 5-nitro-1,10-phenanthroline in water refluxed for 1 h; resulting soln. cooled and filtrated; filtrate evapd. under vac. and residue dissolved in methanol and repptd. into abs. diethyl ether; chromy., NMR; | 90% |

| Conditions | Yield |
|---|---|
| In toluene for 2h; Reflux; | 90% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| Stage #1: 5-Nitro-1,10-phenanthroline; C116H84Cl2Ir2N8 In methanol; dichloromethane for 6h; Reflux; Stage #2: ammonium hexafluorophosphate In methanol; dichloromethane for 0.25h; | 90% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
25148-90-7
N,N'-bis(3-dimethylaminopropyl)oxalamide

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; ethanol addn. of soln. of Cu(NO3)2*3H2O and Et3N (2 equiv.) in MeOH to stirred soln. of (Me2NC3H6NHCO)2 in MeOH; stirring at room temp. for 30 min; filtration, addn. of soln. of Gd(NO3)3*6H2O and 5-NO2-phen (2 equiv.) in EtOH to filtrate, pptn., reflux for 9 h; crystn., filtration, washing several times with MeOH, water and Et2O, drying over P2O5 under reduced pressure, recrystn. from DMF/EtOH (1:3); elem. anal.; | 89% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| Stage #1: 5-Nitro-1,10-phenanthroline; C116H84Cl2Ir2N8 In methanol; dichloromethane for 6h; Reflux; Stage #2: ammonium hexafluorophosphate In methanol; dichloromethane for 0.25h; | 89% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With ethyl orthoformate In ethanol; acetonitrile addn. of a soln. of chromium salt in abs. EtOH to a stirred soln. of piperidinium terephthalate in CH3CN, addn. of ethyl orthoformate, addn. ofa soln. of bpy in abs. EtOH, refluxing for 2 h; filtration, washing with EtOH and Et2O several times, drying over P2O5 in vac., recrystn (DMF); elem. anal.; | 88% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With piperidinium 4-nitrophthalate salt In methanol stirring (room temp., 6 h); ppt. washing (methanol, Et2O), drying (vac.); elem. anal.; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: copper(II) nitrate trihydrate; 4,4,4-trifluoro-1-(2-furanyl)-1,3-butanedione In methanol at 20℃; for 4h; Stage #2: 5-Nitro-1,10-phenanthroline In methanol at 20℃; for 24h; | 88% |

-
-
2999-46-4
Ethyl isocyanoacetate

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
144991-44-6
Ethyl 2H-dibenzoisoindole-1-carboxylate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran for 4h; Ambient temperature; | 87% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 20℃; | 76% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran Ambient temperature; | 53% |
-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
105-56-6
ethyl 2-cyanoacetate

-
-
144991-44-6
Ethyl 2H-dibenzoisoindole-1-carboxylate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran for 8h; Ambient temperature; | 87% |

-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
262278-48-8
C56H31ClN12O12Fe2(2+)*SO4(2-)

| Conditions | Yield |
|---|---|
| In methanol mixt. in MeOH was stirred at room temp. for 6 h under N2; filtered, washed with MeOH and Et2O, dried over P2O5 under reduced pressure, recrystd. from DMF-EtOH; elem. anal.; | 87% |


| Conditions | Yield |
|---|---|
| In ethanol; acetonitrile dropwise addn. of soln. of Ln(ClO4)2 (in EtOH) and sol. of nitrophenanthroline (in MeCN) to soln. of isophthalate (in EtOH) (stirring), refluxing (18 h; pptn.); filtration, washing (EtOH, MeCN, Me2O), drying (over P2O5, vac.), recrystn. (DMF/EtOH 2 : 1); elem. anal.; | A 87% B n/a |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
958242-91-6
[Ir(2-phenylpyridine(-H))2(5-nitro-1,10-phenanthroline)](hexafluorophosphate)

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane (N2); refluxing soln. of iridium compd. and phenanthroline deriv. in 2:1mixt. of CH2Cl2 and methanol for 5-6 h, cooling to room temp., addn. of hexafluorophosphate salt, stirring for 15 min; filtration, evapn., dissolving in CH2Cl2, filtration, layering with ether, cooling to 0°C for overnight, isolation of crystals, elem. anal.; | 87% |


-
-
4199-88-6
5-Nitro-1,10-phenanthroline

-
-
21436-03-3
(S,S)-1,2-diaminocyclohexane

| Conditions | Yield |
|---|---|
| In ethanol; water at 60℃; for 3.5h; | 87% |

5-Nitro-1,10-phenanthroline Chemical Properties
The Molecular Structure of 1,10-Phenanthroline, 5-nitro- (CAS NO.4199-88-6):
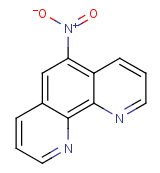
Empirical Formula: C12H7N3O2
Molecular Weight: 225.2029
IUPAC: 5-nitro-1,10-phenanthroline
Appearance: Light yellow to yellow-brown crystalline powder
Nominal Mass: 225 Da
Average Mass: 225.2029 Da
Monoisotopic Mass: 225.053826 Da
Index of Refraction: 1.767
Molar Refractivity: 64.66 cm3
Molar Volume: 155.9 cm3
Surface Tension: 74.8 dyne/cm
Density: 1.444 g/cm3
Flash Point: 222.3 °C
Enthalpy of Vaporization: 67.47 kJ/mol
Boiling Point: 444 °C at 760 mmHg
Vapour Pressure: 1.16E-07 mmHg at 25°C
log P (octanol-water): 2.110
Water Solubility: 27.3 mg/L at 25°C
Atmospheric OH Rate Constant: 2.50E-13 cm3/molecule-sec at 25°C
InChI
InChI=1/C12H7N3O2/c16-15(17)10-7-8-3-1-5-13-11(8)12-9(10)4-2-6-14-12/h1-7H
Smiles
[N+](c1c2c(nccc2)c2c(c1)cccn2)([O-])=O
Synonyms: EINECS 224-097-6
5-Nitro-1,10-phenanthroline Safety Profile
Hazard Codes:  Xi
Xi Xn
Xn
Risk Statements: 36/37/38-40
R36/37/38:Irritating to eyes, respiratory system and skin
R40:Limited evidence of a carcinogenic effect
Safety Statements: 26-36-24/25-22
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36:Wear suitable protective clothing
S24/25:Avoid contact with skin and eyes
S22:Do not breathe dust
WGK Germany: 3
5-Nitro-1,10-phenanthroline Specification
1,10-Phenanthroline, 5-nitro- (CAS NO.4199-88-6) is also called as 5-Nitro-1, 10-diazaphenanthrene ; 5-Nitro-1,10-phenanthroline ; EINECS 224-097-6 ; NSC 4263 .
Related Products
- 5-Nitro-1(2H)-isoquinolinone
- 5-Nitro-1,10-phenanthroline
- 5-Nitro-1,2,3,4-tetrahydroisoquinoline
- 5-Nitro-1,2,3-benzenetricarboxylic acid
- 5-Nitro-1,3-dihydro-2H-benzimidazol-2-one
- 5-Nitro-1,3-dihydroisobenzofuran
- 5-Nitro-1-cyclohexene-1-carboxaldehyde
- 5-Nitro-1H-indole-3-carbaldehyde
- 5-Nitro-1-methyl-1H-indazole
- 5-Nitro-1-naphthonitrile
- 41999-70-6
- 4200-06-0
- 42001-60-5
- 42002-05-1
- 42002-18-6
- 420-03-1
- 420-04-2
- 420118-03-2
- 420-12-2
- 42013-20-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View