-
Name
7-Hydroxycoumarin
- EINECS 202-240-3
- CAS No. 93-35-6
- Article Data241
- CAS DataBase
- Density 1.043 g/cm3
- Solubility Easily soluble in ethanol, chloroform, acetic acid, soluble in dilute alkali, slightly soluble in ether
- Melting Point 230 °C (dec.)(lit.)
- Formula C9H6O3
- Boiling Point 382.081 °C at 760 mmHg
- Molecular Weight 162.145
- Flash Point 181.176 °C
- Transport Information
- Appearance light brownish powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Coumarin, 7-hydroxy- (7CI,8CI);7-Hydroxy-2-chromenone;7-Hydroxy-2H-1-benzopyran-2-one;7-Hydroxy-2H-chromen-2-one;7-Oxycoumarin;Hydrangin;Hydrangine;NSC 19790;Skimmetin;Skimmetine;Umbelliferon;Umbelliferone;
- PSA 50.44000
- LogP 1.49860
Synthetic route
-
-
1516900-23-4
5-hydroxy-2-vinylphenyl acrylate

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 37℃; for 24h; Reagent/catalyst; Solvent; | 99% |
-
-
31005-04-6
7-(benzyloxy)-2H-chromen-2-one

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; ethanethiol In dichloromethane at 30℃; for 72h; | 98.8% |
| With boron trifluoride diethyl etherate; ethanethiol In dichloromethane at 30℃; for 72h; Product distribution; Other reagent: Me2S instead of EtSH. Investigation of the debenzylation of the 4-methyl derivative.; | 98.8% |
| With iodine In 2,2'-[1,2-ethanediylbis(oxy)]bisethanol at 120℃; for 2h; | 70% |
-
-
10387-50-5
7-prenyloxycoumarin

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane at 20℃; for 1h; | 98% |
| at 200℃; |

| Conditions | Yield |
|---|---|
| With Pyridine hydrobromide In sulfolane at 150 - 160℃; for 7h; Inert atmosphere; | 98% |
| With aluminum (III) chloride In toluene for 3h; Reflux; | 86% |
| With 1-n-butyl-3-methylimidazolim bromide; toluene-4-sulfonic acid; 1-butyl-3-methylimidazolium Tetrafluoroborate at 115℃; for 13h; | 80% |
-
-
31005-03-5
7-allyloxycoumarin

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol for 1h; Heating; | 98% |
| With boron trichloride; tetra-(n-butyl)ammonium iodide In dichloromethane at -78 - 20℃; for 2h; dealkylation; | 96% |
| Multi-step reaction with 4 steps 1.1: ozone; DMF / CH2Cl2 / -30 °C 1.2: 62 percent / dimethylsulfide 2.1: rabbit muscle aldolase; (2-hydroxypropyl)-β-cyclodextrin / 48 h / 20 °C / pH 7.5 2.2: 35 percent / acid phoshatase / pH 4.8 3.1: transketolase extract 4.1: bovine serum albumin; Tris buffer / pH 8.2 View Scheme |
-
-
10387-49-2
7-acetyloxycoumarin

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With methanol; zinc | 97% |
| With sodium hydrogen telluride; acetic acid In ethanol for 0.5h; Heating; | 90% |
| With mesoporous silica-supported (Salen) Co(II) catalyst In methanol at 20℃; for 1.5h; chemoselective reaction; | 90% |
| With entrapped lipase-PEG In hexane; isopropyl alcohol at 36℃; Enzyme kinetics; Further Variations:; Reagents; |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.0583333h; Microwave irradiation; | 92% |
| With sulfuric acid at 100℃; for 2.5h; | 74.2% |
| With sulfuric acid at 20 - 100℃; for 3h; von Pechmann Cycloaddition; | 71% |

| Conditions | Yield |
|---|---|
| With ytterbium(III) trifluoromethanesulfonate hydrate at 80℃; for 0.0333333h; Reagent/catalyst; Microwave irradiation; | 92% |
| With para-chlorotoluene; zeolite H-beta at 150℃; for 20h; | 60% |
| With H-BEA In various solvent(s) at 150℃; for 20h; | 60% |
| With iron(III) chloride; silver trifluoromethanesulfonate; trifluoroacetic acid In 1,2-dichloro-ethane at 30℃; for 15h; | 59% |
| With iron(III) chloride; silver trifluoromethanesulfonate In 1,2-dichloro-ethane; trifluoroacetic acid at 30℃; | 55% |
-
-
102-29-4
Resorcinol monoacetate

-
-
292638-85-8
acrylic acid methyl ester

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With formic acid; rhodium(II) acetate dimer; sodium acetate In neat (no solvent) at 100℃; for 3h; Molecular sieve; Inert atmosphere; regioselective reaction; | 92% |
-
-
94739-97-6
7-furoyloxycoumarin

-
A
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
B
-
94740-05-3
8-(Furan-2-carbonyl)-7-hydroxy-chromen-2-one

-
C
-
94740-04-2
6-(Furan-2-carbonyl)-7-hydroxy-chromen-2-one

| Conditions | Yield |
|---|---|
| In ethanol for 48h; Irradiation; | A 90% B 80 mg C 170 % |
| In ethanol for 48h; Irradiation; | A 90 mg B 80 mg C 170 mg |
| In ethanol for 48h; Irradiation; | A 90 mg B 80 mg C 170 mg |


-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With boron trichloride; tetra-(n-butyl)ammonium iodide In dichloromethane at -78℃; for 1h; dealkylation; | 90% |
-
-
80754-21-8
7-(methoxymethoxy)-2H-chromen-2-one

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In neat (no solvent, solid phase) at 20℃; for 0.583333h; Green chemistry; | 90% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With molecular sieve; sodium methylate; triphenylphosphine Wittig reaction; microwave irradiation; | 88% |
| With sodium methylate; magnesium oxide; triphenylphosphine Wittig reaction; | 85% |

| Conditions | Yield |
|---|---|
| With sodium methylate; triphenylphosphine at 80 - 210℃; for 5.5h; Ionic liquid; | 88% |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 100℃; for 0.5h; neat (no solvent); regioselective reaction; | 85% |
| With indium(III) chloride at 90℃; for 2h; Michael addition; | 52% |
| Stage #1: propynoic acid ethyl ester; recorcinol With zinc(II) chloride In 1,4-dioxane for 24h; Reflux; Stage #2: With hydrogenchloride In 1,4-dioxane; water | 40% |
| In 1,4-dioxane for 24h; Reflux; |

| Conditions | Yield |
|---|---|
| With 1-(2-OPPh2-propyl)-3-methylimidazolium hexafluorophosphate; sodium methylate at 110℃; for 0.183333h; Horner-Wadsworth-Emmons olefination; Ionic liquid; Microwave irradiation; | A 84% B 16% |

-
-
471-25-0
Propiolic acid

-
-
108-46-3
recorcinol

-
A
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
B
-
6093-67-0
5-hydroxycoumarin

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In chlorobenzene at 100℃; for 6h; Inert atmosphere; | A 81% B 5% |
| With trifluorormethanesulfonic acid In chlorobenzene at 100℃; for 6h; | A 81% B 5% |
| Dowex 50x2-200 at 120℃; for 0.166667h; microwave irradiation (30 W); | A 69% B 31% |
| Dowex 50x2-200 at 120℃; for 0.166667h; Product distribution; microwave irradiation (30 W); other temperature and reaction time; also without microwave irradiation ; also under reflux in p-ClC6H4CH3 in the presence of Amberlyst; |


| Conditions | Yield |
|---|---|
| With piperidine In ethanol at 20℃; for 0.5h; Knoevenagel Condensation; | 75% |
-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| Stage #1: methyl (triphenylphosphoranylidene)acetate; 2,4-Dihydroxybenzaldehyde In methanol at 20℃; Wittig Olefination; Stage #2: In methanol at 40 - 50℃; for 9h; UV-irradiation; | 74% |
-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With N,N-diethylaniline for 0.25h; Heating; | 70% |
-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

-
A
-
149542-04-1
(E)-3-(2,4-dihydroxyphenyl)acrylic acid ethyl ester

-
B
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| In toluene at 60℃; Wittig reaction; | A 70% B n/a |

-
-
6626-15-9
4-Bromoresorcinol

-
-
292638-85-8
acrylic acid methyl ester

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium hydrogencarbonate at 140℃; for 0.233333h; Heck reaction; Microwave irradiation; neat (no solvent); | 70% |
-
-
31005-03-5
7-allyloxycoumarin

-
A
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
B
-
36914-75-7
7-(2-oxopropoxy)-2H-1-benzopyran-2-one

| Conditions | Yield |
|---|---|
| With oxygen; copper dichloride; palladium dichloride In water; N,N-dimethyl-formamide at 25℃; for 6h; | A n/a B 65% |
-
-
135656-81-4
2-(2,4-Dihydroxy-benzyl)-3,3-dimethyl-pent-4-enoic acid methyl ester

-
A
-
56881-08-4
3-(1,1-dimethylallyl)-7-hydroxycoumarin

-
B
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal In diphenylether Heating; | A 25% B 50% |

-
-
1671-87-0
3,6-di(2'-pyridyl)-1,2,4,5-tetrazine

-
A
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
B
-
36901-11-8
3,6-di(pyridin-2'-yl)-s-tetrazine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 72h; Inert atmosphere; | A 47% B 50% |


-
-
93-35-6
7-hydroxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With 4-(1,2,4,5-tetrazin-3-yl)benzoic acid In water; dimethyl sulfoxide at 37℃; Kinetics; Reagent/catalyst; Irradiation; | 39% |
-
-
471-25-0
Propiolic acid

-
-
108-46-3
recorcinol

-
A
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
B
-
432041-90-2
4,4'-(ethane-1,1-diyl)bis(benzene-1,3-diol)

| Conditions | Yield |
|---|---|
| In water for 21h; Reflux; | A 1% B 27% |


| Conditions | Yield |
|---|---|
| With H-BEA In toluene for 4h; Heating; | 12% |

| Conditions | Yield |
|---|---|
| With H-BEA In toluene for 4h; Heating; | 12% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; zirconium(IV) oxide In water | 5.7% |
| With human liver microsome at 37℃; for 0.25h; Kinetics; Oxidation; Enzymatic reaction; | |
| With NADPH-generating system; recombinant human cytochrome P450 or CYP enzymes; dinoprostone In phosphate buffer pH=7.4; Enzyme kinetics; Further Variations:; Reagents; hydroxylation; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 5h; Heating; | 100% |
| Stage #1: 7-hydroxy-2H-chromen-2-one With potassium carbonate In acetone at 20℃; for 0.0833333h; Stage #2: allyl bromide In acetone for 3h; Reflux; | 100% |
| With potassium carbonate In acetone at 70℃; for 12h; | 98.6% |

| Conditions | Yield |
|---|---|
| In pyridine; dichloromethane for 0.25h; | 100% |
| With SiO2-supported Co(II) Salen complex catalyst at 50℃; for 0.75h; | 99% |
| With pyridine In ethyl acetate at 20℃; for 1.5h; Inert atmosphere; | 99% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
109-64-8
1,3-dibromo-propane

-
-
69150-28-3
7-(3-bromopropoxy)−2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 100% |
| With potassium carbonate In acetonitrile Reflux; | 86.9% |
| With potassium carbonate In acetone Reflux; | 79% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In acetic acid | 100% |
| With palladium 10% on activated carbon; hydrogen In acetic acid at 20℃; for 12h; Inert atmosphere; | 99% |
| With palladium 10% on activated carbon; hydrogen; acetic acid at 50℃; under 2550.26 Torr; for 17h; | 96.5% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
105-36-2
ethyl bromoacetate

-
-
72000-18-1
ethyl 2-((2-oxo-2H-chromen-7-yl)oxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 4h; Heating; | 100% |
| With potassium carbonate In acetone for 5h; Heating; | 98% |
| Stage #1: 7-hydroxy-2H-chromen-2-one With potassium carbonate In acetone for 0.5h; Stage #2: ethyl bromoacetate In ethyl acetate at 20℃; for 6h; | 96% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
4774-14-5
2,6-dichloropyrazine

-
-
894416-91-2
2-chloro-6-(7-coumarinyloxy)-pyrazine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide at 90℃; for 5h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone | 100% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
146900-52-9
3-bromo-7-hydroxy-2H-1-benzopyran-2-one

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; copper(II) chloride monohydrate; zinc(II) chloride In acetonitrile at 20℃; for 0.0833333h; regioselective reaction; | 99.5% |
| With N-Bromosuccinimide at 20℃; for 2h; regioselective reaction; | 92% |
| With γ-picolinium bromochromate In acetonitrile at 90℃; for 1.66667h; regioselective reaction; | 83% |
-
-
17739-45-6, 59146-56-4
2-(2-bromoethoxy)tetrahydropyran

-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
201813-16-3
7-[2-(tetrahydro-2H-2-pyranyloxy)ethoxy]-2H-2-chromenone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; Etherification; | 99% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In N,N-dimethyl-formamide at 50℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 80℃; for 3h; Solvent; | 99% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
100-39-0
benzyl bromide

-
-
31005-04-6
7-(benzyloxy)-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 72h; Heating; | 98% |
| With potassium carbonate In acetone for 4h; Inert atmosphere; Reflux; | 95% |
| Stage #1: 7-hydroxy-2H-chromen-2-one With potassium carbonate In acetone for 0.25h; Inert atmosphere; Reflux; Stage #2: benzyl bromide In acetone Inert atmosphere; Reflux; | 91% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
-
23838-17-7
umbelliprenine

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; dimethyl sulfoxide for 2h; Heating; | 98% |
| With potassium carbonate In acetone at 20℃; for 12h; | 83% |
| With potassium carbonate In acetone for 6h; Reflux; | 80% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
4091-39-8
3-chloro-2-butanone

-
-
156006-08-5
7-((3-oxobutan-2-yl)oxy)-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In acetone at 0 - 60℃; | 98% |
| With potassium carbonate In acetone for 24h; Heating; | 62% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
107-30-2
chloromethyl methyl ether

-
-
80754-21-8
7-(methoxymethoxy)-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide; methyltrialkyl(C8-C10)ammonium chloride (Adogen 464) In dichloromethane for 2h; | 98% |
| Stage #1: 7-hydroxy-2H-chromen-2-one With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; for 3h; Inert atmosphere; Stage #2: chloromethyl methyl ether In tetrahydrofuran; N,N-dimethyl-formamide at 0 - 20℃; for 17h; Inert atmosphere; | 89.2% |
| With sodium hydride 1.) THF, DMF, RT, 3 h, 2.) THF, DMF, RT, 17 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 24h; Reflux; Inert atmosphere; | 98% |
| With potassium hydroxide In acetone for 24h; Inert atmosphere; Reflux; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide for 30h; Heating; | 60% |
| With potassium hydroxide In acetone at 68℃; for 24h; Inert atmosphere; | |
| With potassium hydroxide In acetone at 68℃; for 24h; Inert atmosphere; |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
106-96-7
propargyl bromide

-
-
67268-42-2
7-(propynyloxy)-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 80℃; for 2h; | 98% |
| With potassium carbonate In acetone for 8h; Reflux; | 97% |
| With potassium carbonate In acetone at 50℃; for 18h; | 95% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
535-11-5, 41978-69-2
Ethyl 2-bromopropionate

-
-
314262-30-1
ethyl 2-[(2-oxo-2H-chromen-7-yl)oxy]propanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 5h; Heating; | 98% |
| Stage #1: 7-hydroxy-2H-chromen-2-one With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.333333h; Stage #2: Ethyl 2-bromopropionate In N,N-dimethyl-formamide at 90℃; for 5h; |

| Conditions | Yield |
|---|---|
| With pyridine for 0.5h; Heating; | 98% |
| With pyridine for 0.5h; | 98% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
954390-26-2
tert-butyl (1S,5S)-5-methyl-4-oxocyclohex-2-enyl carbonate

-
-
1232680-29-3
7-((1S,5S)-5-methyl-4-oxocyclohex-2-enyloxy)-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; triphenylphosphine In dichloromethane at 0℃; Inert atmosphere; | 98% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
-
531-59-9
7-methoxycoumarin

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In neat (no solvent) at 100℃; for 0.0666667h; Microwave irradiation; Green chemistry; | 98% |
-
-
93-35-6
7-hydroxy-2H-chromen-2-one

-
-
29527-66-0
7-(3,7-dimethylocta-2,6-dienyloxy)-2H-1-benzopyran-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; for 1h; | 98% |
7-Hydroxycoumarin Specification
The 7-Hydroxycoumarin, with the CAS registry number 93-35-6, is also known as Umbelliferone. It belongs to the product categories of Coumarins; Various Metabolites and Impurities; Intermediates & Fine Chemicals; Metabolites & Impurities; Pharmaceuticals; HU - HZ Environmental Standards; Metabolites Fluorescent Probes, Labels, Particles and Stains; Alphabetic; Fluorescent Labels; H; Other Fluorescent Labels; Pesticides & Metabolites. Its EINECS number is 202-240-3. This chemical's molecular formula is C9H6O3 and molecular weight is 162.14. What's more, its systematic name is 7-Hydroxy-2H-chromen-2-one. Its classification code is Mutation data. This chemical should be sealed and stored in a cool and dry place. It is used as fluorescent indicator and acid-base indicator.
Physical properties of 7-Hydroxycoumarin are: (1)ACD/LogP: 1.58; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.58; (4)ACD/LogD (pH 7.4): 1.46; (5)ACD/BCF (pH 5.5): 9.31; (6)ACD/BCF (pH 7.4): 7.07; (7)ACD/KOC (pH 5.5): 171.70; (8)ACD/KOC (pH 7.4): 130.29; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 46.53 Å2; (13)Index of Refraction: 1.64; (14)Molar Refractivity: 41.648 cm3; (15)Molar Volume: 115.527 cm3; (16)Polarizability: 16.511×10-24cm3; (17)Surface Tension: 59.5 dyne/cm; (18)Density: 1.403 g/cm3; (19)Flash Point: 181.176 °C; (20)Enthalpy of Vaporization: 65.513 kJ/mol; (21)Boiling Point: 382.081 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by 7-acetoxy-chromen-2-one by heating. This reaction will need reagents sodium hydrogen telluride, acetic acid and solvent ethanol with the reaction time of 30 min. The yield is about 90%.
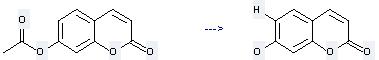
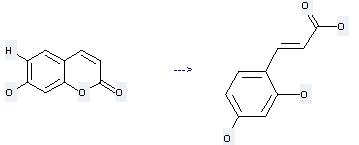
Uses of 7-Hydroxycoumarin: it can be used to produce 2,4-dihydroxy-trans-cinnamic acid at the temperature of 80 °C. It will need reagent aq. KOH with the reaction time of 1 hour. The yield is about 62%.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(cc2c1ccc(=O)o2)O
(2)Std. InChI: InChI=1S/C9H6O3/c10-7-3-1-6-2-4-9(11)12-8(6)5-7/h1-5,10H
(3)Std. InChIKey: ORHBXUUXSCNDEV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 450mg/kg (450mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 18, Pg. 1330, 1968. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View