-
Name
Alpha-Ionone
- EINECS 204-841-6
- CAS No. 127-41-3
- Article Data65
- CAS DataBase
- Density 0.935 g/cm3
- Solubility insoluble in water
- Melting Point 59 - 61oC
- Formula C13H20O
- Boiling Point 257.604 °C at 760 mmHg
- Molecular Weight 192.301
- Flash Point 111.901 °C
- Transport Information
- Appearance clear yellow liquid
- Safety 24/25-26-36/37/39-27
- Risk Codes 42-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Alpha Ionone , Natural;4-(2, 6, 6-Trimethyl-2-cyclohexen-1-yl) 3-buten-2-one;Natural ionone alpha;(E)-(1)-4-(2,6,6-Trimethyl-2-cyclohexen-1-yl)-3-buten-2-one;4-(2,6,6-trimethyl-1-cyclohex-2-enyl)but-3-en-2-one;(E)-4-[(1R)-2,6,6-trimethyl-1-cyclohex-2-enyl]but-3-en-2-one;
- PSA 17.07000
- LogP 3.51410
Synthetic route

| Conditions | Yield |
|---|---|
| With 3-methylbutyric acid; sulfuric acid; N-benzyl-N,N,N-triethylammonium chloride In toluene at -2 - 2℃; for 0.166667h; Reagent/catalyst; Temperature; | 93.8% |
| With benzoic acid |

| Conditions | Yield |
|---|---|
| With butyltriphenylphosphonium dichromate In chloroform for 2h; Oxidation; Heating; | 90% |
-
-
141-10-6
pseudoionone

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

| Conditions | Yield |
|---|---|
| bei der Cyclisierung unter verschiedenen Bedingungen; |

| Conditions | Yield |
|---|---|
| With sodium ethanolate |
-
-
62692-61-9
5E-6,10-dimethylundeca-3,5,9-trien-2-one

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

| Conditions | Yield |
|---|---|
| With lithium perchlorate; tetraethylammonium perchlorate In 1,2-dichloro-ethane at 55℃; for 0.416667h; electrolysis; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
75-52-5
nitromethane

-
-
141-10-6
pseudoionone

-
-
7664-93-9
sulfuric acid

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

| Conditions | Yield |
|---|---|
| at -60 - 60℃; beim Behandeln eines Praeparats von ungewisser konfigurativer Einheitlichkeit; |

-
-
141-10-6
pseudoionone

-
-
7664-93-9
sulfuric acid

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

-
-
141-10-6
pseudoionone

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

-
-
7446-70-0
aluminium trichloride

-
-
141-10-6
pseudoionone

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

-
-
7446-70-0
aluminium trichloride

-
-
141-10-6
pseudoionone

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

-
-
141-10-6
pseudoionone

-
-
7732-18-5
water

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

-
-
141-10-6
pseudoionone

-
-
64-19-7
acetic acid

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

-
-
141-10-6
pseudoionone

-
-
7732-18-5
water

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

-
-
141-10-6
pseudoionone

-
A
-
79-77-6
4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one

-
B
-
127-41-3
alpha-ionone

| Conditions | Yield |
|---|---|
| bei der Cyclisierung durch Saeuren; |

-
-
7664-93-9
sulfuric acid

-
-
13340-72-2, 50911-55-2
4-(2,6,6-trimethyl-cyclohex-2-enyl)-but-3-en-2-one oxime

-
-
127-41-3
alpha-ionone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium wire View Scheme |

| Conditions | Yield |
|---|---|
| In methanol Irradiation; | A 95 %Spectr. B n/a |
-
-
127-41-3
alpha-ionone

| Conditions | Yield |
|---|---|
| With Fe(bpmen)(OTf)2; dihydrogen peroxide; acetic acid In acetonitrile at 20℃; Concentration; | 100% |
-
-
127-41-3
alpha-ionone

-
-
1538551-03-9
C13H22O

| Conditions | Yield |
|---|---|
| With iron(III)-acetylacetonate; phenylsilane In ethanol at 60℃; for 0.25h; | 98% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 92% |


| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 90% |


| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 89% |


| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 86% |

-
-
127-41-3
alpha-ionone

-
-
31499-72-6
7,8-dihydro-α-ionone

| Conditions | Yield |
|---|---|
| With sodium dithionite; 2-methyldecanal; sodium hydrogencarbonate In 1,4-dioxane at 50℃; | 85% |
| With ethanol; hydrogen; palladium | |
| With hydrogen In tetrahydrofuran; methanol at 20℃; under 760.051 Torr; |
-
-
127-41-3
alpha-ionone

-
-
190059-33-7
(E)-4-(1,3,3-Trimethyl-7-oxa-bicyclo[4.1.0]hept-2-yl)-but-3-en-2-one

| Conditions | Yield |
|---|---|
| With potassium superoxide; 2-Nitrobenzenesulfonyl chloride In acetonitrile at -35℃; for 3h; | 85% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 85% |

-
-
32806-04-5
diethyl methoxymethylphosphonic acid

-
-
127-41-3
alpha-ionone

| Conditions | Yield |
|---|---|
| Stage #1: diethyl methoxymethylphosphonic acid With potassium tert-butylate In N,N-dimethyl-formamide at -20℃; for 1.5h; Inert atmosphere; Stage #2: alpha-ionone In N,N-dimethyl-formamide at -20℃; for 1.5h; Reagent/catalyst; Solvent; Temperature; Wittig-Horner Reaction; Inert atmosphere; | 83% |
-
-
127-41-3
alpha-ionone

-
-
631-61-8
ammonium acetate

-
-
126-81-8
dimedone

-
-
139-85-5
3,4-dihydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 81% |


| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 80% |


| Conditions | Yield |
|---|---|
| Stage #1: 2-Trifluoromethylbenzaldehyde; alpha-ionone With cetyltrimethylammonim bromide In water for 0.5h; Aldol condensation; Stage #2: With sodium hydroxide In water at 20℃; for 24h; | 77% |
| sodium hydroxide In water |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 77% |


| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 75% |


| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 75% |


| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 110℃; for 3h; | 72% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120 - 130℃; for 2h; Sealed tube; | 72% |

-
-
127-41-3
alpha-ionone

-
-
37677-81-9
4-(1,3,3-trimethyl-7-oxabicyclo[4.1.0]hept-2-yl)-3-buten-2-one

| Conditions | Yield |
|---|---|
| Stage #1: alpha-ionone With N-Bromosuccinimide; dimethyl sulfoxide at 10℃; for 0.5h; Inert atmosphere; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In dimethyl sulfoxide at 0℃; for 0.5h; Inert atmosphere; | 70% |
-
-
67-56-1
methanol

-
-
127-41-3
alpha-ionone

-
-
127256-04-6
1,1-dimethoxy-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one

| Conditions | Yield |
|---|---|
| With ammonium persulfate; diphenyl diselenide for 3h; Heating; | 65% |

| Conditions | Yield |
|---|---|
| With chloro[5,10,15,20-tetrakis(4-dimethylamino-2,3,5,6-tetrafluorophenyl)porphyrinate]iron(III) In 1,2-dichloro-ethane at 25 - 35℃; Irradiation; Molecular sieve; Sealed tube; Inert atmosphere; | 60% |

| Conditions | Yield |
|---|---|
| Stage #1: alpha-ionone; m-tolyl aldehyde With cetyltrimethylammonim bromide In water for 0.5h; Aldol condensation; Stage #2: With sodium hydroxide In water at 20℃; for 24h; | 55% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Trifluoromethylbenzaldehyde; alpha-ionone With cetyltrimethylammonim bromide In water for 0.5h; Aldol condensation; Stage #2: With sodium hydroxide In water at 20℃; for 24h; | 53% |

| Conditions | Yield |
|---|---|
| Stage #1: alpha-ionone; 3-nitro-benzaldehyde With cetyltrimethylammonim bromide In water for 0.5h; Aldol condensation; Stage #2: With sodium hydroxide In water at 20℃; for 24h; | 53% |

| Conditions | Yield |
|---|---|
| Stage #1: alpha-ionone; 2-Fluorobenzaldehyde With cetyltrimethylammonim bromide In water for 0.5h; Aldol condensation; Stage #2: With sodium hydroxide In water at 20℃; for 24h; | 52% |

| Conditions | Yield |
|---|---|
| Stage #1: alpha-ionone; 3-Trifluoromethylbenzaldehyde With cetyltrimethylammonim bromide In water for 0.5h; Aldol condensation; Stage #2: With sodium hydroxide In water at 20℃; for 24h; | 38% |

| Conditions | Yield |
|---|---|
| Stage #1: alpha-ionone; 2-fluoro-5-trifluoromethylbenzaldehyde With cetyltrimethylammonim bromide In water for 0.5h; Aldol condensation; Stage #2: With sodium hydroxide In water at 20℃; for 24h; | 36% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-propan-1-one With 1-ethyl-piperidine; tin(II) trifluoromethanesulfonate In dichloromethane for 0.25h; Inert atmosphere; Stage #2: alpha-ionone In dichloromethane Inert atmosphere; | 30% |
Alpha-Ionone Specification
The Alpha-Ionone, with the CAS registry number 127-41-3, is also known as (3E)-4-(2,6,6-Trimethyl-2-cyclohexen-1-yl)-3-buten-2-one. It belongs to the product categories of Biochemistry; Monocyclic Monoterpenes; Terpenes; Intermediates & Fine Chemicals; Pharmaceuticals; Building Blocks; C13 to C14; Carbonyl Compounds; Chemical Synthesis; Citrus Aurantium (Seville orange); Ginkgo Biloba; Ketones; Nutrition Research; Organic Building Blocks; Phytochemicals by Plant (Food/Spice/Herb). Its EINECS registry number is 204-841-6. This chemical's molecular formula is C13H20O and molecular weight is 192.3. What's more, its IUPAC name is called (E)-4-(2,6,6-Trimethylcyclohex-2-en-1-yl)but-3-en-2-one. It should be stored in a cool, dry and well-ventilated place. Alpha-Ionone is an aroma compound commonly found in essential oils such as rose oil. It is a degradation products of caratenoids produced by caratenoid cleavage dioxygenases (CCD).
Physical properties about Alpha-Ionone are: (1)ACD/LogP: 3.66; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.66; (4) ACD/LogD (pH 7.4): 3.66; (5)ACD/BCF (pH 5.5): 356.12; (6)ACD/BCF (pH 7.4): 356.12; (7)ACD/KOC (pH 5.5): 2333.67; (8)ACD/KOC (pH 7.4): 2333.67; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.512; (14)Molar Refractivity: 61.708 cm3; (15)Molar Volume: 205.659 cm3; (16)Polarizability: 24.463×10-24cm3; (17)Surface Tension: 32.772 dyne/cm; (18)Density: 0.935 g/cm3; (19)Flash Point: 111.901 °C; (20)Enthalpy of Vaporization: 49.517 kJ/mol; (21)Boiling Point: 257.604 °C at 760 mmHg; (22)Vapour Pressure: 0.014 mmHg at 25 °C.
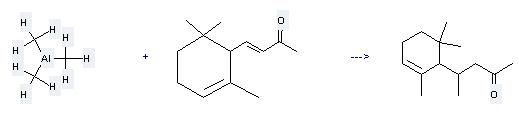
Uses of Alpha-Ionone: it is used to produce other chemicals. For example, it can react with trimethylaluminium to get 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-2-pentanone. This reaction needs reagent CuBr and solvents hexane, tetrahydrofuran. The reaction time is 2 hours. The yield is 90 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin or other mucous membranes and may cause damage to health. And it is irritating to eyes, respiratory system and skin. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In addition, you should avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(\C=C\C1C(=C/CCC1(C)C)\C)C
(2) InChI: InChI=1S/C13H20O/c1-10-6-5-9-13(3,4)12(10)8-7-11(2)14/h6-8,12H,5,9H2,1-4H3/b8-7+
(3) InChIKey: UZFLPKAIBPNNCA-BQYQJAHWSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 2277mg/kg (2277mg/kg) | FAO Nutrition Meetings Report Series. Vol. 44A, Pg. 46, 1967. |
Related Products
- Alpha-Ionone
- 127420-27-3
- 127-42-4
- 127425-73-4
- 127-43-5
- 127437-44-9
- 127446-35-9
- 127464-43-1
- 127464-60-2
- 127476-27-1
- 127-47-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View