-
Name
Avobenzone
- EINECS 274-581-6
- CAS No. 70356-09-1
- Article Data66
- CAS DataBase
- Density 1.079 g/cm3
- Solubility 27μg/L at 20℃
- Melting Point 81-84 °C
- Formula C20H22O3
- Boiling Point 463.6 °C at 760 mmHg
- Molecular Weight 310.393
- Flash Point 203.1 °C
- Transport Information
- Appearance white powder
- Safety 60-61
- Risk Codes 50/53
-
Molecular Structure
-
Hazard Symbols
 N
N
- Synonyms 1-(4-Methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione;1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl)propan-1,3-dione;4-(1,1-Dimethylethyl)-4'-methoxydibenzoylmethane;4-Methoxy-4'-tert-butyldibenzoylmethane;4-tert-Butyl-4'-methoxydibenzoylmethane;1,3-Propanedione,1-[4-(1,1-dimethylethyl)phenyl]-3-(4-methoxyphenyl)-;Butylmethoxydibenzoylmethane;Escalol 517;Eusolex 9020;NeoHeliopan 357;Parsol 1789;Parsol A;Parsol RTM 1789;Photoplex;
- PSA 43.37000
- LogP 4.44840
Synthetic route
-
-
26537-19-9
methyl 4-tert-butylbenzoate

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With potassium methanolate In toluene at 110℃; under 750.075 Torr; for 2h; Product distribution / selectivity; | 95% |
| With potassium methanolate; sodium methylate In methanol; toluene at 105 - 108℃; for 3.5h; Temperature; | 92.8% |
| With sodium amide In 5,5-dimethyl-1,3-cyclohexadiene at 95 - 100℃; for 5h; Concentration; | 78% |
| Stage #1: 1-(4-methoxyphenyl)ethanone With sodium amide In toluene at 80℃; for 0.25h; Claisen Condensation; Stage #2: methyl 4-tert-butylbenzoate In toluene at 80℃; for 6h; Claisen Condensation; Stage #3: With hydrogenchloride In water; toluene at 20℃; Claisen Condensation; | 67% |
| With sodium hydride In tetrahydrofuran at 60℃; for 18h; Claisen condensation; Inert atmosphere; | 48% |
-
-
92279-83-9
tert-butyl 4-(tert-butyl)benzoate

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With potassium methanolate In toluene under 750.075 Torr; for 1.75h; Product distribution / selectivity; Heating; | 91% |
-
-
258497-50-6
3-((4-t-butyl)phenyl)-1-(4-methoxyphenyl)-2-propen-1-one

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 3-((4-t-butyl)phenyl)-1-(4-methoxyphenyl)-2-propen-1-one With palladium(II) trifluoroacetate In toluene at 40 - 50℃; for 7h; Stage #2: With tert.-butylhydroperoxide In toluene; tert-butyl alcohol for 1.5h; | 89.1% |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium methylate; chlorine In methanol; 5,5-dimethyl-1,3-cyclohexadiene | 85% |
-
-
874-90-8
4-methoxybenzonitrile

-
-
30095-47-7
2-bromo-1-(4-(tert-butyl)phenyl)ethanone

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxybenzonitrile; 2-bromo-1-(4-(tert-butyl)phenyl)ethanone With chloro-trimethyl-silane; zinc In 1,4-dioxane for 6h; Blaise Reaction; Reflux; Inert atmosphere; Stage #2: With hydrogenchloride In 1,4-dioxane; water at 100℃; for 0.5h; pH=2; Blaise Reaction; Inert atmosphere; | 70% |
-
-
133931-49-4
(Z)-1-(4-tert-Butyl-phenyl)-3-hydroxy-3-(4-methoxy-phenyl)-propenone

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile Product distribution; Irradiation; determination by NMR; | |
| In hexane at 25℃; Kinetics; Equilibrium constant; | |
| In hexane for 3h; Quantum yield; UV-irradiation; |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| In methanol at 35℃; for 0.166667h; Product distribution / selectivity; UVB irradiation; |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| In methanol at 35℃; for 0.166667h; Product distribution / selectivity; UVB irradiation; |
-
-
1262100-39-9
1,1-(4-tert-butyl-benzoyl)(4'-methoxybenzoyl)butane

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| In acetonitrile at 21.84℃; Activation energy; Kinetics; Quantum yield; Mechanism; Reagent/catalyst; Temperature; Norrish type II reaction; UV-irradiation; |
-
-
955359-34-9
3-(4-tert-butylphenyl)-3-hydroxy-1-(4-methoxyphenyl)-propan-1-one

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| UV-irradiation; |
-
-
175086-57-4
difluoroboron avobenzone

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| at 22.84℃; Kinetics; Temperature; | |
| With water In dimethyl sulfoxide Kinetics; |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide for 2h; Irradiation; |
-
-
108-88-3
toluene

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: aluminum (III) chloride / 1.42 h / 23.5 °C 2: potassium permanganate / 8.5 h / 85 °C 3: methanesulfonic acid / 8 h / Reflux 4: sodium amide / 5,5-dimethyl-1,3-cyclohexadiene / 5 h / 95 - 100 °C View Scheme |
-
-
98-51-1
4-tert-butyltoluene

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium permanganate / 8.5 h / 85 °C 2: methanesulfonic acid / 8 h / Reflux 3: sodium amide / 5,5-dimethyl-1,3-cyclohexadiene / 5 h / 95 - 100 °C View Scheme |
-
-
98-73-7
4-(1,1-dimethylethyl)benzoic acid

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: methanesulfonic acid / 8 h / Reflux 2: sodium amide / 5,5-dimethyl-1,3-cyclohexadiene / 5 h / 95 - 100 °C View Scheme |
-
-
943-27-1
4'-t-butylacetophenone

-
-
121-98-2
methyl 4-methoxybenzoate

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 4'-t-butylacetophenone With sodium amide In tetrahydrofuran at 20℃; for 1h; Stage #2: methyl 4-methoxybenzoate In tetrahydrofuran at 50 - 60℃; for 2h; |
-
-
943-27-1
4'-t-butylacetophenone

-
-
94-30-4
ethyl 4-methoxybenzoate

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 4.5h; Reflux; |
-
-
93-55-0
1-phenyl-propan-1-one

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; copper diacetate In 1,2-dichloro-benzene at 120℃; for 24h; Inert atmosphere; Schlenk technique; | 97% |
-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In dimethyl sulfoxide at 20 - 65℃; | 95% |
| With N-Bromosuccinimide; Dithioglycoluril In methanol at 20℃; for 0.0833333h; | 80% |
| With N-Bromosuccinimide In neat (no solvent) for 0.833333h; | 76% |
-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In dimethyl sulfoxide at 20℃; for 0.666667h; | 92% |
-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In dimethyl sulfoxide at 20℃; for 2h; | 90% |
-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

-
-
132944-34-4
1-(4-(tert-butyl)phenyl)-3-(4-hydroxyphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at -78 - 20℃; | 89% |
| With hydrogen bromide; acetic acid In water for 16h; Inert atmosphere; Reflux; | 62.9% |
| With Hexanethiol; potassium tert-butylate In N,N-dimethyl-formamide at 5 - 110℃; |
-
-
12354-84-6, 12354-85-7
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With potassium hydroxide In methanol at 20℃; for 1h; Stage #2: bis[dichloro(pentamethylcyclopentadienyl)iridium(III)] In methanol at 20℃; for 24h; | 89% |
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With potassium hydroxide In methanol at 20℃; for 1h; Stage #2: bis[dichloro(pentamethylcyclopentadienyl)iridium(III)] In methanol at 20℃; for 24h; | 76% |
-
-
75073-97-1
dimethyl 2-(4-methoxyphenyl)cyclopropane-1,1-dicarboxylate

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) In dichloromethane at 25℃; for 24h; | 89% |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With potassium hydroxide In methanol at 20℃; for 1h; Stage #2: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer In methanol at 20℃; for 24h; | 86% |
-
-
20621-29-8
trifluoromethylsulfinyl chloride

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 0.183333h; Inert atmosphere; | 84% |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With sodium carbonate In 2-ethoxy-ethanol for 24h; Reflux; | 81% |
-
-
17084-13-8
potassium hexafluorophosphate

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: bis(2,2'-bipyridine)dichlororuthenium(II) dihydrate; 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With triethylamine In ethanol; water at 100℃; for 2h; Sealed tube; Inert atmosphere; Stage #2: potassium hexafluorophosphate In ethanol; water | 81% |

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With potassium hydroxide In methanol at 20℃; for 1h; Stage #2: [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In methanol at 20℃; for 20h; | 80% |
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With potassium hydroxide In methanol at 20℃; for 1h; Stage #2: [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In methanol at 20℃; for 24h; | 79% |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With potassium hydroxide In methanol at 20℃; for 1h; Stage #2: [(hexamethylbenzene)RuCl2]2 In methanol at 20℃; for 20h; | 74% |
-
-
106-54-7
p-Chlorothiophenol

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With rose bengal; sodium iodide In acetonitrile at 20℃; for 48h; Irradiation; | 74% |
-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

-
-
1393380-37-4
3-(4-tert-butylphenyl)-5-(4-methoxyphenyl)-1H-pyrazole

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol for 3h; Reflux; | 72% |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With sodium carbonate In 2-ethoxy-ethanol for 24h; Reflux; | 69% |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With sodium carbonate In 2-ethoxy-ethanol for 24h; Reflux; | 69% |
-
-
67-64-1
acetone

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In water at 50℃; for 2h; Schlenk technique; Green chemistry; | 68% |
-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

-
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 40 - 50℃; Inert atmosphere; | 67% |
-
-
17084-13-8
potassium hexafluorophosphate

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: [Ru(4,7-diphenyl-1,10-phenanthroline)2Cl2]; 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With triethylamine In ethanol; water at 100℃; for 2h; Sealed tube; Inert atmosphere; Stage #2: potassium hexafluorophosphate In ethanol; water | 65% |

-
-
106-96-7
propargyl bromide

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With potassium carbonate In acetone at 20℃; for 0.5h; Stage #2: propargyl bromide In acetone at 20℃; | 61% |
-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

-
-
175086-57-4
difluoroboron avobenzone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 60℃; for 1h; Inert atmosphere; | 59% |

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione With α,α,α-trifluorotoluene; C20H21Cl3IrNO; zinc trifluoromethanesulfonate; sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate at 20℃; for 0.0833333h; Inert atmosphere; Glovebox; Stage #2: 2,2,2,-trichloroethoxycarbonyl azide at 10℃; for 24h; Inert atmosphere; Sealed tube; chemoselective reaction; | 59% |
-
-
140-88-5
ethyl acrylate

-
-
70356-09-1
1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione

-
A
-
25305-58-2
ethyl 5-(4-methoxyphenyl)-5-oxopentanoate

-
B
-
101577-33-7
ethyl 5-(4-(tert-butyl)phenyl)-5-oxopentanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 85℃; Sealed tube; | A 57% B 40% |

Avobenzone Chemical Properties
IUPAC Name: 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione
Product Categories: Organics; cosmetic raw material; UV-Absorber
Molecular Structure of Avobenzone (CAS NO.70356-09-1):
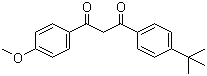
Molecular Formula: C20H22O3
Molecular Weight: 310.39 g/mol
EINECS: 274-581-6
Index of Refraction: 1.545
Surface Tension: 38.7 dyne/cm
Density: 1.079 g/cm3
Flash Point: 203.1 °C
Enthalpy of Vaporization: 72.49 kJ/mol
Boiling Point: 463.6 °C at 760 mmHg
Vapour Pressure: 8.93E-09 mmHg at 25 °C
Melting Point: 81-84 °C
Appearance: white crystal
XLogP3-AA: 4.8
H-Bond Acceptor: 3
Rotatable Bond Count: 6
Tautomer Count: 3
Exact Mass: 310.156895
MonoIsotopic Mass: 310.156895
Topological Polar Surface Area: 43.4
Heavy Atom Count: 23
Canonical SMILES: CC(C)(C)C1=CC=C(C=C1)C(=O)CC(=O)C2=CC=C(C=C2)OC
InChI: InChI=1S/C20H22O3/c1-20(2,3)16-9-5-14(6-10-16)18(21)13-19(22)15-7-11-17(23-4)12-8-15/h5-12H,13H2,
1-4H3
InChIKey: XNEFYCZVKIDDMS-UHFFFAOYSA-N
Avobenzone History
Avobenzone was patented in 1973 and was approved in the EU in 1978. It was approved by the FDA in 1988. Its use is approved world wide.
Avobenzone Uses
Avobenzone (CAS NO.70356-09-1) is used for whitening and sunscreen. It is also used for the protection of skin products in the production of cosmetics.
Avobenzone Safety Profile
Hazard Codes:  N
N
Risk Statements: 50/53
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 60-61
S60:This material and its container must be disposed of as hazardous waste.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN3077 9/PG 3
HS Code: 29145000
Avobenzone Specification
Avobenzone (CAS NO.70356-09-1) is also named as 1,3-Propanedione, 1-(4-(1,1-dimethylethyl)phenyl)-3-(4-
methoxyphenyl)- ; 1-(4-(1,1-Dimethylethyl)phenyl)-3-(4-methoxyphenyl)-1,3-propanedione ; 1-(p-tert-Butylphenyl)-3-(p-methoxyphenyl)-1,3-propanedione ; Avobenzona ; Avobenzona [INN-Spanish] ; Avobenzonum ; Avobenzonum [INN-Latin] ; HSDB 7423 ; Parsol 1789 ; UNII-G63QQF2NOX . It is a dibenzoylmethane derivative. Avobenzone is sensitive to the properties of the solvent, being relatively stable in polar protic solvents and unstable in nonpolar environments. Avobenzone reacts with minerals to form colored complexes. It also reacts with boron trifluoride to form a stable crystalline complex that is highly fluorescent under UV irradiation.
Related Products
- Avobenzone
- 7035-68-9
- 703-59-3
- 70359-30-7
- 70359-46-5
- 7036-04-6
- 70360-76-8
- 70361-61-4
- 703-61-7
- 70362-41-3
- 70362-46-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View