-
Name
Bendamustine hydrochloride
- EINECS 631-540-0
- CAS No. 3543-75-7
- Article Data18
- CAS DataBase
- Density
- Solubility
- Melting Point 149-151 °C
- Formula C16H22Cl3N3O2
- Boiling Point 585.2 °C at 760 mmHg
- Molecular Weight 394.729
- Flash Point 307.7 °C
- Transport Information
- Appearance pale brown crystals
- Safety 36-37
- Risk Codes 60-61-22-40
-
Molecular Structure
- Hazard Symbols T,Xn
- Synonyms 1H-Benzimidazole-2-butanoicacid, 5-[bis(2-chloroethyl)amino]-1-methyl-, monohydrochloride (9CI);2-Benzimidazolebutyric acid, 5-[bis(2-chloroethyl)amino]-1-methyl-,monohydrochloride (8CI);1H-Benzimidazole-2-butanoicacid, 5-[bis(2-chloroethyl)amino]-1-methyl-, hydrochloride (1:1);Cytostasan;IMET 3393;Ribomustin;SDX 105;ZIMET 33/93;Bendamustine HCl;
- PSA 58.36000
- LogP 4.06660
Synthetic route

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 4h; Reflux; | 83% |
| With hydrogenchloride; water at 40℃; for 2h; Product distribution / selectivity; | |
| With hydrogenchloride In water at 25 - 28℃; for 0.25h; |
-
-
3543-74-6
4-{5-[bis-(2-hydroxyl-ethyl)-amino]-1-methyl-1H-benzoimidazol-2yl}-butyric acid ethyl ester

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-{5-[bis-(2-hydroxyl-ethyl)-amino]-1-methyl-1H-benzoimidazol-2yl}-butyric acid ethyl ester With thionyl chloride In dichloromethane at -1 - 22℃; for 17h; Stage #2: With hydrogenchloride In water at 75℃; | 76.2% |
| Stage #1: 4-{5-[bis-(2-hydroxyl-ethyl)-amino]-1-methyl-1H-benzoimidazol-2yl}-butyric acid ethyl ester With thionyl chloride In chloroform at 0 - 5℃; Stage #2: With hydrogenchloride; pyrographite In water at 30℃; Reflux; | 75% |
| Stage #1: 4-{5-[bis-(2-hydroxyl-ethyl)-amino]-1-methyl-1H-benzoimidazol-2yl}-butyric acid ethyl ester With thionyl chloride In chloroform at 0 - 25℃; Large scale; Stage #2: With hydrogenchloride In chloroform; water Large scale; | 75% |
-
-
109882-25-9
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid methyl ester

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 4h; Reflux; Large scale reaction; | 76% |
| With hydrogenchloride; water for 4h; Reflux; | |
| With hydrogenchloride In water at 25 - 28℃; for 0.25h; |
-
-
1313020-25-5
bendamustine isopropyl ester

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water at 35 - 40℃; for 1.5h; Product distribution / selectivity; | 75% |
-
-
1313020-26-6
isopropyl 4-(5-amino-1-methyl-1H-benzo[d]imidazol-2-yl)butanoate

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / water / 2 h / 70 - 75 °C 1.2: 1.5 h / 70 - 75 °C 2.1: thionyl chloride / dichloromethane / 7 h / 0 - 30 °C 3.1: hydrogenchloride; water / 1.5 h / 35 - 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 2.1: thionyl chloride / dichloromethane / 2 h / 25 - 60 °C 2.2: 25 - 30 °C View Scheme |
-
-
1221157-09-0
4-(2,4-dinitrophenylcarbamoyl)butyric acid methyl ester

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium carbonate / acetonitrile / 23 - 25 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 3: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 4: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / acetonitrile / 23 - 25 °C 2.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 2.2: 2 h / 60 - 65 °C 3.1: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 4.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / acetonitrile / 23 - 25 °C 2.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 2.2: 2 h / 60 - 65 °C 3.1: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 4.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 4 steps 1: potassium carbonate / acetonitrile / 23 - 25 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 3: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 4: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme |
-
-
1221157-10-3
4-[(2,4-dinitrophenyl)methylcarbamoyl]butyric acid methyl ester

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 2: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 3: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 3 steps 1.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 1.2: 2 h / 60 - 65 °C 2.1: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 3.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 3 steps 1.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 1.2: 2 h / 60 - 65 °C 2.1: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 3.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 3 steps 1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 2: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 3: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme |
-
-
1221157-11-4
4-(5-amino-1-methyl-1H-benzoimidazol-2-yl)butyric acid methyl ester hydrochloride

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 2: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 2 steps 1: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 2: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2: potassium carbonate / acetonitrile / 23 - 25 °C 3: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 4: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 5: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 5 steps 1.1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2.1: potassium carbonate / acetonitrile / 23 - 25 °C 3.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 3.2: 2 h / 60 - 65 °C 4.1: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 5.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 5 steps 1.1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2.1: potassium carbonate / acetonitrile / 23 - 25 °C 3.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 3.2: 2 h / 60 - 65 °C 4.1: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 5.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 5 steps 1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2: potassium carbonate / acetonitrile / 23 - 25 °C 3: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 4: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 5: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2: potassium carbonate / acetonitrile / 23 - 25 °C 3: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 4: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 5: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 5 steps 1.1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2.1: potassium carbonate / acetonitrile / 23 - 25 °C 3.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 3.2: 2 h / 60 - 65 °C 4.1: sodium tetrahydroborate / 1,2-dichloro-ethane / 30 - 50 °C / Large scale reaction 5.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 5 steps 1.1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2.1: potassium carbonate / acetonitrile / 23 - 25 °C 3.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 3.2: 2 h / 60 - 65 °C 4.1: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 5.1: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme | |
| Multi-step reaction with 5 steps 1: toluene / 6 h / 95 - 105 °C / Large scale reaction 2: potassium carbonate / acetonitrile / 23 - 25 °C 3: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2068.65 Torr / Large scale reaction 4: borane-THF / tetrahydrofuran / 30 - 50 °C / Large scale reaction 5: hydrogenchloride / 4 h / Reflux; Large scale reaction View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium sulfide; sodium hydrogencarbonate / water; methanol / 25 - 80 °C 2.1: 35 - 40 °C 2.2: 80 - 85 °C 2.3: 25 - 30 °C 3.1: hydrogen / Raney nickel / methanol / 25 - 30 °C / 4654.46 - 5171.62 Torr 4.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 5.1: thionyl chloride / dichloromethane / 25 - 60 °C 5.2: 55 - 60 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium sulfide; sodium hydrogencarbonate / methanol; water / 25 - 80 °C 2.1: 35 - 40 °C 2.2: 35 - 85 °C / Reflux 3.1: hydrogen / methanol / 4654.46 - 5171.62 Torr / Autoclave 4.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 5.1: thionyl chloride / dichloromethane / 2 h / 25 - 60 °C 5.2: 25 - 30 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: water; methanol / 0 - 30 °C 2.1: sodium sulfide; sodium hydrogencarbonate / water; methanol / 25 - 80 °C 3.1: 35 - 40 °C 3.2: 80 - 85 °C 3.3: 25 - 30 °C 4.1: hydrogen / Raney nickel / methanol / 25 - 30 °C / 4654.46 - 5171.62 Torr 5.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 6.1: thionyl chloride / dichloromethane / 25 - 60 °C 6.2: 55 - 60 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: methanol; water 2.1: sodium sulfide; sodium hydrogencarbonate / methanol; water / 25 - 80 °C 3.1: 35 - 40 °C 3.2: 35 - 85 °C / Reflux 4.1: hydrogen / methanol / 4654.46 - 5171.62 Torr / Autoclave 5.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 6.1: thionyl chloride / dichloromethane / 2 h / 25 - 60 °C 6.2: 25 - 30 °C View Scheme |
-
-
41939-61-1
N1-methyl-4-nitrobenzene-1,2-diamine

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 35 - 40 °C 1.2: 80 - 85 °C 1.3: 25 - 30 °C 2.1: hydrogen / Raney nickel / methanol / 25 - 30 °C / 4654.46 - 5171.62 Torr 3.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 4.1: thionyl chloride / dichloromethane / 25 - 60 °C 4.2: 55 - 60 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: 35 - 40 °C 1.2: 35 - 85 °C / Reflux 2.1: hydrogen / methanol / 4654.46 - 5171.62 Torr / Autoclave 3.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 4.1: thionyl chloride / dichloromethane / 2 h / 25 - 60 °C 4.2: 25 - 30 °C View Scheme |
-
-
1313020-24-4
isopropyl 4-(5-bis(2-hydroxyethyl)amino-1-methyl-1H-benzo[d]imidazol-2-yl)butanoate

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: isopropyl 4-(5-bis(2-hydroxyethyl)amino-1-methyl-1H-benzo[d]imidazol-2-yl)butanoate With thionyl chloride In dichloromethane at 25 - 60℃; Stage #2: With hydrogenchloride In water at 55 - 60℃; | |
| Stage #1: isopropyl 4-(5-bis(2-hydroxyethyl)amino-1-methyl-1H-benzo[d]imidazol-2-yl)butanoate With thionyl chloride In dichloromethane at 25 - 60℃; for 2h; Stage #2: With water In dichloromethane at 25 - 30℃; | 16.4 g |
-
-
1374784-01-6
isopropyl 4-(1-methyl-5-nitro-1H-benzo[d]imidazol-2-yl)butanoate

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogen / Raney nickel / methanol / 25 - 30 °C / 4654.46 - 5171.62 Torr 2.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 3.1: thionyl chloride / dichloromethane / 25 - 60 °C 3.2: 55 - 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: hydrogen / methanol / 4654.46 - 5171.62 Torr / Autoclave 2.1: N-ethyl-N,N-diisopropylamine / water / 25 - 95 °C 3.1: thionyl chloride / dichloromethane / 2 h / 25 - 60 °C 3.2: 25 - 30 °C View Scheme |
-
-
109882-31-7
4-{5-[bis-(2-hydroxyethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}-butanoic acid methyl ester

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trichlorophosphate / 0.25 h / Reflux 2: hydrogenchloride / water / 0.25 h / 25 - 28 °C View Scheme | |
| Stage #1: 4-{5-[bis-(2-hydroxyethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}-butanoic acid methyl ester With thionyl chloride In dichloromethane at 20 - 30℃; for 7h; Stage #2: With hydrogenchloride In dichloromethane at 20 - 70℃; |
-
-
3543-72-4
4‐(1‐methyl‐5‐nitro‐1H‐benzoimidazol‐2‐yl)butyric acid ethyl ester

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: iron; hydrogenchloride; ammonium chloride / methanol; water / 2 h / 60 - 65 °C 2.1: sodium carbonate; sodium iodide / 65 - 70 °C 3.1: thionyl chloride / dichloromethane / 0 °C / Reflux 3.2: 80 - 90 °C View Scheme | |
| Multi-step reaction with 4 steps 1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 2: potassium carbonate / water / 13.5 h / 69 - 70 °C / pH 4.2 - 5.5 / Large scale 3: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 4: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 4 steps 1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 2: diammonium hydrogenphosphate / water / 65 - 70 °C / pH 4.2 - 5.5 3: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 4: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: 5% Pd/C-iron; hydrogen / ethanol / 3000.3 Torr 2.1: potassium iodide; diammonium phosphate / water / 65 - 70 °C / pH 4.2 - 5.5 3.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 3.2: 75 °C View Scheme |
-
-
3543-73-5
ethyl 4‐(5‐amino‐1‐methyl‐1H‐benzo[d]imidazol‐2‐yl)butanoate

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium carbonate; sodium iodide / 65 - 70 °C 2.1: thionyl chloride / dichloromethane / 0 °C / Reflux 2.2: 80 - 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / water / 13.5 h / 69 - 70 °C / pH 4.2 - 5.5 / Large scale 2: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 3: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 3 steps 1: diammonium hydrogenphosphate / water / 65 - 70 °C / pH 4.2 - 5.5 2: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 3: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium iodide; diammonium phosphate / water / 65 - 70 °C / pH 4.2 - 5.5 2.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 2.2: 75 °C View Scheme | |
| Multi-step reaction with 3 steps 1: acetic acid / water / -5 - 0 °C 2: thionyl chloride / dichloromethane / 2 h / -5 - 20 °C 3: hydrogenchloride / water / 4 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: toluene / 4 h / 70 - 85 °C 2.1: water / 3 h / 75 °C 2.2: 55 - 60 °C 3.1: sulfuric acid / 10 h / Reflux 4.1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 5.1: potassium carbonate / water / 13.5 h / 69 - 70 °C / pH 4.2 - 5.5 / Large scale 6.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 7.1: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: toluene / 4 h / 70 - 85 °C 2.1: water / 3 h / 75 °C 2.2: 55 - 60 °C 3.1: sulfuric acid / 10 h / Reflux 4.1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 5.1: diammonium hydrogenphosphate / water / 65 - 70 °C / pH 4.2 - 5.5 6.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 7.1: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: hydrogenchloride / toluene / 4 h / 85 °C 1.2: 3 h / 75 °C 2.1: sulfuric acid / 10 h / Reflux 3.1: 5% Pd/C-iron; hydrogen / ethanol / 3000.3 Torr 4.1: potassium iodide; diammonium phosphate / water / 65 - 70 °C / pH 4.2 - 5.5 5.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 5.2: 75 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: toluene / 4 h / 70 - 85 °C 2.1: water / 3 h / 75 °C 2.2: 55 - 60 °C 3.1: sulfuric acid / 10 h / Reflux 4.1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 5.1: potassium carbonate / water / 13.5 h / 69 - 70 °C / pH 4.2 - 5.5 / Large scale 6.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 7.1: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: toluene / 4 h / 70 - 85 °C 2.1: water / 3 h / 75 °C 2.2: 55 - 60 °C 3.1: sulfuric acid / 10 h / Reflux 4.1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 5.1: diammonium hydrogenphosphate / water / 65 - 70 °C / pH 4.2 - 5.5 6.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 7.1: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: hydrogenchloride / toluene / 4 h / 85 °C 1.2: 3 h / 75 °C 2.1: sulfuric acid / 10 h / Reflux 3.1: 5% Pd/C-iron; hydrogen / ethanol / 3000.3 Torr 4.1: potassium iodide; diammonium phosphate / water / 65 - 70 °C / pH 4.2 - 5.5 5.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 5.2: 75 °C View Scheme |
-
-
31349-48-1
4-(1-methyl-5-nitro-1H-benzimidazol-2-yl)butanoic acid

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: sulfuric acid / 10 h / Reflux 2: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 3: potassium carbonate / water / 13.5 h / 69 - 70 °C / pH 4.2 - 5.5 / Large scale 4: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 5: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 5 steps 1: sulfuric acid / 10 h / Reflux 2: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 3: diammonium hydrogenphosphate / water / 65 - 70 °C / pH 4.2 - 5.5 4: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 5: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: sulfuric acid / 10 h / Reflux 2.1: 5% Pd/C-iron; hydrogen / ethanol / 3000.3 Torr 3.1: potassium iodide; diammonium phosphate / water / 65 - 70 °C / pH 4.2 - 5.5 4.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 4.2: 75 °C View Scheme |

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; acetone at 40 - 75℃; | 225 g |
-
-
451459-95-3
5-(2-fluoro-5-nitroanilino)-5-oxopentanoic acid

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: water / 3 h / 75 °C 1.2: 55 - 60 °C 2.1: sulfuric acid / 10 h / Reflux 3.1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 4.1: potassium carbonate / water / 13.5 h / 69 - 70 °C / pH 4.2 - 5.5 / Large scale 5.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 6.1: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: water / 3 h / 75 °C 1.2: 55 - 60 °C 2.1: sulfuric acid / 10 h / Reflux 3.1: ferrous(II) sulfate heptahydrate; hydrogen; Iron(III) nitrate nonahydrate; 5% Pd/C / ethanol / 3000.3 Torr 4.1: diammonium hydrogenphosphate / water / 65 - 70 °C / pH 4.2 - 5.5 5.1: thionyl chloride / dichloromethane / 17 h / -1 - 22 °C 6.1: hydrogenchloride / water; acetone / 40 - 75 °C View Scheme |
-
-
2016-57-1
1-aminodecane

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-14-4
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}-N-decyl-butyramide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 2.7 - 10℃; for 1.5h; Inert atmosphere; | 100% |
-
-
112-53-8
1-dodecyl alcohol

-
-
3543-75-7
bendamustine hydrochloride

-
-
1456608-94-8
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid dodecyl ester

| Conditions | Yield |
|---|---|
| Stage #1: bendamustine hydrochloride With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 0.733333h; Inert atmosphere; Stage #2: 1-dodecyl alcohol In dichloromethane for 0.183333h; Inert atmosphere; Stage #3: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 23.5h; Reagent/catalyst; | 98% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 70℃; for 1h; Inert atmosphere; | 97% |
-
-
67-56-1
methanol

-
-
3543-75-7
bendamustine hydrochloride

-
-
109882-25-9
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid methyl ester

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 65℃; for 1h; Inert atmosphere; | 94% |
-
-
3543-75-7
bendamustine hydrochloride

-
-
16506-27-7
bendamustine

| Conditions | Yield |
|---|---|
| With triethylamine In methanol other reagent; | 89% |
-
-
112-30-1
1-Decanol

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-04-2
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid decyl ester

| Conditions | Yield |
|---|---|
| Stage #1: bendamustine hydrochloride With triethylamine In dichloromethane at 20℃; for 0.25h; Inert atmosphere; Stage #2: 1-Decanol With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane at 20℃; for 20h; Reagent/catalyst; Time; Inert atmosphere; | 88.6% |
-
-
3543-75-7
bendamustine hydrochloride

-
-
1221157-14-7
4-(5-(bis(2-chloroethyl)amino)-1-methyl-1H-benzo[d]imidazol-2-yl)butan-1-ol

| Conditions | Yield |
|---|---|
| With borane-THF In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 4h; Ambient temperature; | 85% |
-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| With 1-hydroxy-pyrrolidine-2,5-dione; hydrazine hydrate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride for 12h; | 82.4% |
-
-
3543-75-7
bendamustine hydrochloride

-
-
109882-29-3
4-<1-Methyl-5-benzimidazolyl-(2)>butansaeure-hydrochlorid

| Conditions | Yield |
|---|---|
| With water at 95℃; for 5h; | 82% |
-
-
688009-09-8
4-amino-1-((2R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)-3,3-difluoro-tetrahydrofuran-2-yl)pyrimidin-2(1H)-one

-
-
3543-75-7
bendamustine hydrochloride

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 40℃; for 12h; | 79% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 16h; Inert atmosphere; | 73% |

| Conditions | Yield |
|---|---|
| Stage #1: bendamustine hydrochloride With triethylamine; dicyclohexyl-carbodiimide In chloroform at 0℃; for 0.5h; Stage #2: irinotecan With dmap In chloroform at 0 - 20℃; for 48h; Darkness; | 69% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-hydroxysulfosuccinimide sodium salt In water at 20℃; for 12.5h; | 66.1% |
-
-
2491-20-5
L-alanine methyl ester hydrochloride

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-17-7
(S)-2-(4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyrylamino)propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 1.9 - 20℃; for 4h; Inert atmosphere; | 63.3% |

| Conditions | Yield |
|---|---|
| Stage #1: bendamustine hydrochloride With dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: 5-Fluoro-2'-deoxyuridine With dmap; triethylamine In N,N-dimethyl-formamide at 25℃; for 48h; Darkness; | 61% |
-
-
2016-42-4
tetradecylamine

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-15-5
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}-N-tetradecyl-butyramide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 3.3 - 20℃; for 3h; Inert atmosphere; | 57.6% |
-
-
3543-75-7
bendamustine hydrochloride

-
-
879126-98-4, 879126-99-5, 191732-72-6
Lenalidomide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 40℃; | 51% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 48.3% |
-
-
661-19-8
1-docosanol

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-09-7
4-{5-[bis-(chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butyric acid docosyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; Inert atmosphere; | 43.1% |
-
-
10203-28-8
rac-2-dodecanol

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-10-0
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid 2-dodecyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; Inert atmosphere; | 40% |
-
-
112-72-1
1-Tetradecanol

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-05-3
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid tetradecyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 20h; Inert atmosphere; | 36.4% |
-
-
3543-75-7
bendamustine hydrochloride

-
-
108-93-0
cyclohexanol

-
-
1609623-12-2
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid cyclohexyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 18h; Inert atmosphere; | 27.9% |
-
-
5205-34-5
decan-5-ol

-
-
3543-75-7
bendamustine hydrochloride

-
-
1609623-11-1
4-{5-[bis-(2-chloro-ethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid 5-decyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In 1,2-dichloro-ethane at 75℃; for 120h; Inert atmosphere; | 24.2% |
-
-
3543-75-7
bendamustine hydrochloride

-
A
-
109882-29-3
4-<1-Methyl-5-benzimidazolyl-(2)>butansaeure-hydrochlorid

-
B
-
109882-26-0
4-(6-((2-chloroethyl)(2-hydroxyethyl)amino)-3-methylbenzimidazolyl(2))butyric acid hydrochloride

| Conditions | Yield |
|---|---|
| With water at 95℃; for 1h; |
Bendamustine hydrochloride Chemical Properties
The molecular structure of Bendamustine hydrochloride (CAS NO.3543-75-7):
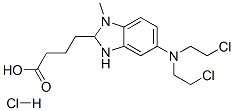
IUPAC Name: 4-[5-[Bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid hydrochloride
Molecular Weight: 394.72378 g/mol
Molecular Formula: C16H22Cl3N3O2
Melting Point: 149-151 °C
Flash Point: 307.7 °C
Enthalpy of Vaporization: 91.95 kJ/mol
Boiling Point: 585.2 °C at 760 mmHg
Vapour Pressure: 1.55E-14 mmHg at 25 °C
H-Bond Donor: 2
H-Bond Acceptor: 4
Rotatable Bond Count: 9
Tautomer Count: 2
Exact Mass: 393.07776
MonoIsotopic Mass: 393.07776
Topological Polar Surface Area: 58.4
Heavy Atom Count: 24
Canonical SMILES: CN1C2=C(C=C(C=C2)N(CCCl)CCCl)N=C1CCCC(=O)O.Cl
InChI: InChI=1S/C16H21Cl2N3O2.ClH/c1-20-14-6-5-12(21(9-7-17)10-8-18)11-13(14)19-15(20)3-2-4-16(22)23;/h5-6,11H,2-4,7-10H2,1H3,(H,22,23);1H
InChIKey: ZHSKUOZOLHMKEA-UHFFFAOYSA-N
Product Categories: API intermediates; Intermediates & Fine Chemicals; Pharmaceuticals
Bendamustine hydrochloride Uses
Bendamustine hydrochloride (CAS NO.3543-75-7) is used as an anticancer drug.
Bendamustine hydrochloride Toxicity Data With Reference
| 1. | orl-mus TDLo:250 mg/kg/4D-I:CAR | ARGEAR Archiv fuer Geschwulstforschung. 43 (1974),16. | ||
| 2. | ipr-mus TDLo:50 mg/kg/4D-I:CAR | ARGEAR Archiv fuer Geschwulstforschung. 43 (1974),16. | ||
| 3. | orl-rat LD50:200 mg/kg | ATSUDG Archives of Toxicology, Supplement. 8 (1985),504. | ||
| 4. | ivn-rat LD50:40 mg/kg | ATSUDG Archives of Toxicology, Supplement. 8 (1985),504. | ||
| 5. | orl-mus LD50:250 mg/kg | ARGEAR Archiv fuer Geschwulstforschung. 43 (1974),16. | ||
| 6. | ipr-mus LD50:100 mg/kg | ARGEAR Archiv fuer Geschwulstforschung. 43 (1974),16. | ||
| 7. | ivn-mus LD50:80 mg/kg | ATSUDG Archives of Toxicology, Supplement. 8 (1985),504. |
Bendamustine hydrochloride Safety Profile
A poison by ingestion, intravenous, and intraperitoneal routes. Questionable carcinogen with experimental carcinogenic and teratogenic data. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of HCl and NOx.
Bendamustine hydrochloride Specification
Bendamustine hydrochloride (CAS NO.3543-75-7) is also named as Bendamustin hydrochloride ; Bendamustine HCl ; CCRIS 1864 ; Cytostasan ; IMET 3393 ; NSC 138783 ; Ribomustin ; SDX 105 ; Treanda ; UNII-981Y8SX18M ; gamma(1-Methyl-5-bis(beta-chloraethyl)aminobenzimidazoyl-2)buttersaeurehydrochlorid ; gamma(1-Methyl-5-bis(beta-chloraethyl)aminobenzimidazoyl-2)buttersaeurehydrochlorid [German] . Bendamustine hydrochloride (CAS NO.3543-75-7) is pale brown crystals.
Related Products
- Bendamustine
- Bendamustine
- Bendamustine hydrochloride
- 354-38-1
- 35438-57-4
- 35439-70-4
- 3544-24-9
- 3544-25-0
- 35444-44-1
- 35445-70-6
- 35446-36-7
- 35447-68-8
- 35447-75-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View