-
Name
Benzil
- EINECS 205-157-0
- CAS No. 134-81-6
- Article Data1182
- CAS DataBase
- Density 1.165 g/cm3
- Solubility Soluble in ethanol, ethyl ether, chloroform, ethyl acetate, benzene, toluene, nitrobenzene. Insoluble in water
- Melting Point 94-95 °C(lit.)
- Formula C14H10O2
- Boiling Point 347 °C at 760 mmHg
- Molecular Weight 210.232
- Flash Point 142.559 °C
- Transport Information
- Appearance yellow crystals or powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Diphenylethanedione;Diphenylglyoxal;Esacure KBO;NSC 220315;NSC 4041;Wy20910;Benzil;Benzil(8CI);Ethanedione, diphenyl- (9CI);1,2-Diphenylethane-1,2-dione;1,2-Diphenylethanedione;Bibenzoyl;Dibenzoyl;Diphenyl diketone;Diphenyl-a,b-diketone;
- PSA 34.14000
- LogP 2.75220
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine chromium peroxide In benzene for 0.1h; Product distribution; Heating; effect of various chromium(VI) based oxidants; | 100% |
| With pyridine chromium peroxide In benzene for 0.1h; Heating; | 100% |
| With 4-dimethylaminopyridine tribromide In dichloromethane for 0.25h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With sodium periodate; sulfuric acid; C31H29Br2N3Ru*CH2Cl2 In water; acetonitrile at 25℃; for 0.5h; Inert atmosphere; Schlenk technique; | 100% |
| With sodium periodate; sulfuric acid; ruthenium-carbon composite In water; acetonitrile at 25℃; for 0.5h; Catalytic behavior; Reagent/catalyst; Temperature; Time; Solvent; Green chemistry; | 100% |
| With SO3-dioxane at 60℃; for 0.25h; | 99% |
-
-
16939-18-7
1,2-diphenyl-2-thioxoethanone

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In diethyl ether for 1h; oxidation with nitric acid; | 100% |
-
-
26205-39-0
1,2-diphenyl-2-(trimethylsilyloxy)ethanone

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With copper nitrate - dinitrogen tetroxide adduct In tetrachloromethane for 0.75h; Oxidation; Heating; | 100% |
| With aluminium trichloride; silver bromate In acetonitrile for 0.4h; Heating; | 98% |
| With Montmorillonite K10; ferric nitrate Oxidation; deprotection; Irradiation; | 95% |
-
-
180295-26-5
(2RS)-(+/-)-2-<<(1,1-dimethylethyl)dimethylsilyl>oxy>-1,2-diphenylethanone

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With copper nitrate - dinitrogen tetroxide adduct In tetrachloromethane for 1h; Oxidation; Heating; | 100% |
| With N-hydroxyphthalimide; oxygen; cobalt(II) benzoate In acetonitrile at 20℃; for 9h; | 93% |
| With manganese(IV) oxide; aluminium trichloride In acetonitrile for 0.333333h; Oxidation; Heating; | 40% |

-
A
-
932703-85-0
[(6-Ph2TPA)Ni(O2CPh)]ClO4

-
B
-
33868-50-7
phenacyl benzoate

-
C
-
201230-82-2
carbon monoxide

-
D
-
134-81-6
benzil

-
E
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With oxygen | A 100% B n/a C n/a D n/a E n/a |


-
A
-
33868-50-7
phenacyl benzoate

-
B
-
201230-82-2
carbon monoxide

-
D
-
134-81-6
benzil

-
E
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With oxygen In acetonitrile at 20℃; | A n/a B n/a C 100% D 12% E 11 mg |

-
-
492-70-6, 655-48-1, 2325-10-2, 38270-73-4, 52340-78-0, 579-43-1
1,2-diphenyl-1,2-ethanediol

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With Me2Se*NCS; 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene 1.)1h 0 deg C, 1h room temperature, 2.) 4h room temperature; | 99% |
| With octahydro-2,5-epiminopentalen-7-yloxidanyl; acetic acid; sodium nitrite In acetonitrile at 20℃; for 4h; air; | 99% |
| With 9-benzyl-9-norazaadamantane N-oxyl; oxygen; acetic acid; sodium nitrite In acetonitrile at 20℃; for 4h; Catalytic behavior; Concentration; Reagent/catalyst; Solvent; Temperature; Time; | 99.3% |

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-2'-hydroxy-diphenyl-2-methylethanone With potassium chloride at 60 - 76℃; for 4.33h; Stage #2: With dimethyltitanocene; isopentyl butanoate for 4.5h; Temperature; | 99.3% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; dimethyl sulfoxide at 55℃; for 24h; | 99% |
| With C14H14N6O2; oxygen; sodium acetate; palladium diacetate at 120℃; under 760.051 Torr; for 48h; | 97% |
| With potassium hydrogencarbonate; dimethyl sulfoxide at 80℃; | 96% |
-
-
32368-19-7
1,2-diphenyl-2-methylthio-1-ethanone

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With CuCl2*2H2O In water; acetone for 1h; Heating; | 99% |
-
-
119-53-9
2-hydroxy-2-phenylacetophenone

-
-
88-74-4
2-nitro-aniline

-
A
-
1684-14-6
2,3-diphenylquinoxaline

-
B
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With sodium hydroxide In toluene at 120℃; for 3h; Inert atmosphere; Sealed tube; | A 99% B 50% |


| Conditions | Yield |
|---|---|
| With antimonypentachloride; dimethyl sulfoxide In nitromethane; benzene for 2h; Heating; | A n/a B 98.7% |

| Conditions | Yield |
|---|---|
| With oxygen; silica gel; 4-aminoperbenzoic acid In dichloromethane at 20℃; for 9h; | 98% |
| With graphite oxide In chloroform at 120℃; for 24h; | 17% |
| With oxygen; graphite oxide In chloroform at 120℃; for 24h; Sealed tube; | 17% |
| With selenium(IV) oxide at 200℃; | |
| Multi-step reaction with 2 steps 1: SeO2 / 200 °C / sowie auf 300grad 2: SeO2 / 200 °C View Scheme |
-
-
78576-73-5
2,2'-Diphenyl-[2,2']bi[[1,3]dithiolanyl]

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With t-butyl bromide; dimethyl sulfoxide at 70 - 75℃; for 4h; | 98% |
| With trimethylsilyl iodide; dimethyl sulfoxide In tetrachloromethane at 75 - 80℃; for 6h; | 98% |
| With fluorosulfonylchloride In diethyl ether; water Ambient temperature; | 86% |
-
-
91493-19-5
4-iodoxybiphenyl

-
-
501-65-5
diphenyl acetylene

-
A
-
1591-31-7
4-iodo-biphenyl

-
B
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| In nitrobenzene at 170℃; for 5h; | A 98% B 34.5% |


| Conditions | Yield |
|---|---|
| With (2 equiv); triphenylantimony dibromide; triethylamine In chloroform-d1 for 17h; Product distribution; Mechanism; reactions of α-hydroxyketones and esters in presence of var. bases; | A 98% B n/a |

| Conditions | Yield |
|---|---|
| In acetonitrile; trifluoroacetic acid excess diphenylacetylene was treated with cis-((Cn*)(CF3CO2)Ru(VI)O2)ClO4 in 0.2M CF3COOH/MeCN soln. at room temp.; recrystn. from MeCN/Et2O; elem. anal.; | A 92% B 98% |


| Conditions | Yield |
|---|---|
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In N,N-dimethyl-formamide at 23 - 25℃; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; Oxone In water at 20℃; for 24h; | 98% |
| With iodine; dimethyl sulfoxide; copper(ll) bromide for 8h; Catalytic behavior; Reagent/catalyst; Schlenk technique; Heating; | 95% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; acetic acid; copper(I) bromide at 100℃; for 2h; Mechanism; Reagent/catalyst; Temperature; | 90% |
-
-
574-15-2, 574-16-3, 14090-77-8
benzilmonoxime

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With bis(1-CH2Ph-3,5,7-3N-1-N(1+)tricyclo[3.3.1.13,7]decaneS2O8 In acetonitrile for 0.333333h; Oxidation; Heating; | 97% |
| With KMnO4/alumina at 50℃; for 0.5h; | 97% |
| With Cu(NO3)2-SiO2 for 0.133333h; oxime cleavage; microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With ruthenium(IV) oxide; sodium periodate In dichloromethane; water; acetonitrile for 0.5h; Ambient temperature; | A 97% B n/a |

| Conditions | Yield |
|---|---|
| With Cu(NO3)2-SiO2 for 0.0833333h; Oxidation; oxime cleavage; microwave irradiation; | 97% |
| With water; Dess-Martin periodane In dichloromethane at 5 - 20℃; for 0.333333h; | 92% |
| With quinolinium monofluorochromate(VI) In dichloromethane for 5h; Product distribution; Oxidation; Heating; | 85% |
| With imidazolium fluorochromate In acetonitrile at 20℃; for 0.5h; Oxidation; | 79% |
-
-
24242-77-1
α-iodo-α-phenylacetophenone

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide at 100℃; for 2h; | 97% |
| With dimethyl sulfoxide at 100℃; for 2h; | 97% |
| With dimethyl sulfoxide at 130℃; for 1h; | 90% |
-
-
52340-78-0
(R,R)-hydroxybenzoin

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With oxone; C18H17IN2O7PolS(1-)*Na(1+); tetra(n-butyl)ammonium hydrogensulfate In acetonitrile at 70℃; for 18h; Reagent/catalyst; Solvent; Sealed tube; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| With water; iodine; oxygen In acetonitrile at 140℃; for 20h; Reagent/catalyst; Solvent; Temperature; Sealed tube; chemoselective reaction; | 96% |
| With tert.-butylhydroperoxide; [Ru2Cl(3,6-bis(1-(2-pyridylmethyl)imidazol-2-yliden-3-yl)pyridazine)(CH3CN)4](PF6)3; sodium iodide In water; acetonitrile at 20℃; for 0.166667h; Catalytic behavior; Reagent/catalyst; Solvent; | 92% |
| With tert.-butylhydroperoxide; (p-cymene)ruthenium(II) chloride; tetrabutylammonium iodide In water; toluene; acetonitrile at 20℃; for 1h; | 91% |

| Conditions | Yield |
|---|---|
| With 4-methoxypyridine; lithium carbonate In N,N-dimethyl acetamide at 25℃; for 24h; Irradiation; | 96% |
| With 4-methoxypyridine; tris(2,2'-bipyridine)ruthenium dichloride; lithium carbonate In N,N-dimethyl acetamide at 25℃; for 24h; Irradiation; | 81% |
| With Amberlyst A-26; nitrate form In benzene for 2h; Heating; | 80% |
-
-
30416-76-3
(benzoylphenylmethylene)triphenylphosphorane

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With potassium permanganate; magnesium sulfate In toluene at 50 - 60℃; for 20h; | 96% |
| With N-Sulfonyloxaziridine 3 In dichloromethane at 25℃; for 0.5h; | 76% |
-
-
7510-34-1, 10496-80-7, 6313-26-4
(Z)-1,2,3,4-tetraphenyl-2-butene-1,4-dione

-
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| With formic acid; sulfuric acid for 0.0333333h; microwave irradiation; | 96% |

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((2S,4S,8R)-8-vinylquinuclidin-2-yl)methyl)urea In chloroform at 24℃; Concentration; Reagent/catalyst; Solvent; | 95% |
| With 4-acetylamino-2,2,6,6-tetramethylpiperidine-N-oxyl; toluene-4-sulfonic acid In dichloromethane at 18℃; 1.) 0 deg C, 1 h, 2.) room temp., 72 h; | 88% |
| With aluminium(III) phenoxide; p-benzoquinone; benzene |

| Conditions | Yield |
|---|---|
| at 100℃; for 0.25h; | 100% |
| With gallium(III) triflate In ethanol at 20℃; for 0.0833333h; | 100% |
| With aluminum oxide at 20℃; for 0.166667h; neat (no solvent); | 100% |

| Conditions | Yield |
|---|---|
| With gallium(III) triflate In acetonitrile at 20℃; for 0.0833333h; Reactivity; Solvent; Time; Concentration; | 100% |
| With PEG-400 at 110℃; for 0.166667h; Neat (no solvent); | 100% |
| With zirconium(IV) chloride In methanol at 20℃; for 0.0833333h; | 100% |

| Conditions | Yield |
|---|---|
| With gallium(III) triflate In ethanol at 20℃; for 0.0833333h; | 100% |
| With 1,1,1,3',3',3'-hexafluoro-propanol at 20℃; for 1h; | 92% |
| With acetic acid |

| Conditions | Yield |
|---|---|
| at 160℃; for 0.25h; | 100% |
| H6P2W18O62 In acetic acid at 20℃; for 0.0833333h; | 98% |
| at 120℃; for 0.0583333h; Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With titanium(III) chloride In acetone at 0℃; for 1h; | 100% |
| Stage #1: benzil With sodium tetrahydroborate at 70℃; for 1.5h; Ball milling; neat (no solvent); Stage #2: With water regiospecific reaction; | 100% |
| With hydrogen; palladium In methanol at 20℃; under 3800 Torr; for 46h; | 98% |

| Conditions | Yield |
|---|---|
| With third generation polystyrene supported poly(amidoamine) dendrimer In ethanol at 50℃; for 2h; Knoevenagel condensation; | 100% |
| With N,N-dimethyl-aniline In ethanol | |
| With potassium carbonate In ethanol for 8h; Reflux; |
-
-
134-81-6
benzil

-
-
121-45-9
phosphorous acid trimethyl ester

-
-
4850-55-9
2,2,2-trimethoxy-4,5-diphenyl-2,2-dihydro-1,3,2-dioxaphospholene

| Conditions | Yield |
|---|---|
| 100% | |
| In benzene |

| Conditions | Yield |
|---|---|
| With titanium(III) chloride In acetone at 0℃; for 1h; Product distribution; Mechanism; various reaction conditions; | 100% |
| With titanium(III) chloride In acetone at 0℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With titanium(III) chloride In acetone at 40℃; for 1h; | 100% |
-
-
132635-33-7
4,5-Diamino-6-(2-hydroxy-ethylamino)-2H-pyridazin-3-one

-
-
64-19-7
acetic acid

-
-
134-81-6
benzil

-
-
133219-06-4
Acetic acid 2-(8-oxo-2,3-diphenyl-7,8-dihydro-pyrazino[2,3-d]pyridazin-5-ylamino)-ethyl ester

| Conditions | Yield |
|---|---|
| for 6h; Heating; | 100% |


| Conditions | Yield |
|---|---|
| With titanium(III) chloride In acetone at 0℃; for 1h; | 100% |
-
-
132635-37-1
4,5-Diamino-6-(2-hydroxy-2-phenyl-ethylamino)-2-methyl-2H-pyridazin-3-one

-
-
134-81-6
benzil

-
-
133219-13-3
8-(2-Hydroxy-2-phenyl-ethylamino)-6-methyl-2,3-diphenyl-6H-pyrazino[2,3-d]pyridazin-5-one

| Conditions | Yield |
|---|---|
| In acetic acid Ambient temperature; | 100% |
-
-
134-81-6
benzil

-
-
36239-19-7
<1,2-2H2>-1,2-diphenyl-1,2-ethanediol

| Conditions | Yield |
|---|---|
| With sodium borodeuteride In methanol for 1h; Ambient temperature; | 100% |
| With sodium borodeuteride; water-d2 In tetrahydrofuran at 0℃; for 1h; | 100% |
| With lithium aluminium deuteride In diethyl ether at 20℃; for 4h; | 9.7 g |

| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)oxovanadium; oxygen; acetic acid at 80℃; under 760.051 Torr; for 8h; Sealed tube; | 100% |
| With dihydrogen peroxide; sodium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 1h; | 99% |
| With Oxone In water; acetonitrile for 48h; Reflux; | 97% |
-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
134-81-6
benzil

-
-
13362-56-6
6,7-dimethyl-2,3-diphenylquinoxaline

| Conditions | Yield |
|---|---|
| With aluminum oxide at 20℃; for 0.166667h; neat (no solvent); | 100% |
| With amberlyst-15 In water at 70℃; for 0.2h; | 99% |
| In benzene for 2h; cyclocondensation; Heating; | 97% |

| Conditions | Yield |
|---|---|
| Product distribution; Further Variations:; Temperatures; in melt; solid-state reaction; | 100% |
| at 20℃; for 1h; solid-state reaction; |
-
-
5416-80-8
2-methyl-1H-indole-3-carbaldehyde

-
-
134-81-6
benzil

-
-
662150-78-9
3-(4,5-diphenyl-1H-imidazol-2-yl)-2-methyl-1H-indole

| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid Reflux; | 100% |
| With ammonium acetate In acetic acid | |
| With ammonium acetate In acetic acid for 3 - 5h; Heating / reflux; | |
| With ammonium acetate In ethanol for 5h; Reflux; |
-
-
4426-21-5
oxybis(diphenylborane)

-
-
1670-14-0
benzamidine monohydrochloride

-
-
134-81-6
benzil

-
-
115438-41-0
1,3,3,5,7-pentaphenyl-2,4-dioxa-8-aza-6-azonia-3-boratabicyclo{3.3.3}oct-6-ene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol room temp.; crystn. from reaction mixt., washing (ethanol, ether), or evapn., extraction (hot ethanol), crystn. on cooling; elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid Reflux; | 100% |
| With ammonium acetate In neat (no solvent) for 0.233333h; Microwave irradiation; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: (S)-1-phenylethanol With sec.-butyllithium In diethyl ether; hexane Stage #2: benzil In diethyl ether; hexane at -40 - 20℃; for 24h; Inert atmosphere; | 100% |
-
-
86-81-7
3,4,5-trimethoxy-benzaldehyde

-
-
134-81-6
benzil

-
-
57-13-6
urea

-
-
190780-24-6
4,5‐diphenyl‐2‐(3,4,5‐trimethoxyphenyl)‐1H‐imidazole

| Conditions | Yield |
|---|---|
| With ammonium acetate; zinc(II) chloride at 70 - 110℃; for 0.5h; Green chemistry; | 100% |


| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Sealed tube; Schlenk technique; | 100% |
Benzil Consensus Reports
Benzil Specification
The Benzil, with the CAS registry number 134-81-6, is also known as Diphenylethanedione. It belongs to the product categories of Pharmaceutical Intermediates; Intermediates; Functional Materials; Photopolymerization Initiators; Highly Purified Reagents; Other Categories; Zone Refined Products; Bioactive Small Molecules; Building Blocks; C13 to C14; Carbonyl Compounds; Cell Biology; Chemical Synthesis; Ketones; Organic Building Blocks. Its EINECS number is 205-157-0. This chemical's molecular formula is C14H10O2 and molecular weight is 210.23. What's more, its systematic name is Benzil. Its classification code is Skin / Eye Irritant. Benzil is commonly used as pharmaceutical intermediates, insecticide and curing agent. It is used in organic synthesis. Its main use is as a photoinitiator in polymer chemistry. It is used in the free-radical curing of polymer networks.
Physical properties of Benzil are: (1)ACD/LogP: 3.38; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.38; (4)ACD/LogD (pH 7.4): 3.38; (5)ACD/BCF (pH 5.5): 218.17; (6)ACD/BCF (pH 7.4): 218.17; (7)ACD/KOC (pH 5.5): 1643.31; (8)ACD/KOC (pH 7.4): 1643.31; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 34.14 Å2; (13)Index of Refraction: 1.594; (14)Molar Refractivity: 61.247 cm3; (15)Molar Volume: 180.379 cm3; (16)Polarizability: 24.28×10-24cm3; (17)Surface Tension: 47.4 dyne/cm; (18)Density: 1.165 g/cm3; (19)Flash Point: 142.559 °C; (20)Enthalpy of Vaporization: 59.123 kJ/mol; (21)Boiling Point: 347 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
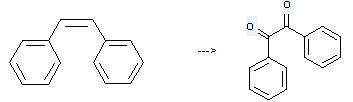
Preparation: this chemical can be prepared by cis-1,2-diphenyl-ethene at the temperature of 120 °C. This reaction will need reagent benzeneseleninic anhydride and solvent chlorobenzene with the reaction time of 8 hours. The yield is about 89%.
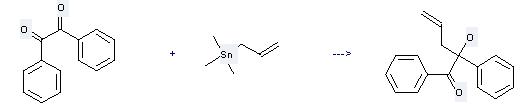
Uses of Benzil: it can be used to produce 2-hydroxy-1,2-diphenyl-pent-4-en-1-one at the ambient temperature. It will need solvent acetonitrile with the reaction time of 5 hours. The yield is about 94%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: c1ccc(cc1)C(=O)C(=O)c2ccccc2
(2)Std. InChI: InChI=1S/C14H10O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10H
(3)Std. InChIKey: WURBFLDFSFBTLW-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | > 3gm/kg (3000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 15, Pg. 359, 1984. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View