-
Name
Benzoic anhydride
- EINECS 202-291-1
- CAS No. 93-97-0
- Article Data391
- CAS DataBase
- Density 1.211 g/cm3
- Solubility water: 0.01 g/L
- Melting Point 38-42 °C(lit.)
- Formula C14H10O3
- Boiling Point 360 °C at 760 mmHg
- Molecular Weight 226.232
- Flash Point 168.4 °C
- Transport Information
- Appearance white to almost white crystals or crystalline
- Safety 26-39-37/39
- Risk Codes 37/38-41-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzoicacid, anhydride (9CI);Benzoic anhydride (8CI);Benzoylbenzoate;NSC 37116;Benzoic anhydride;
- PSA 43.37000
- LogP 2.68380
Synthetic route

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate In toluene for 3h; Heating; | 99% |
| With pyridine; 1,1,1-trichloro-3,3,3-trifluoro-propan-2-one; water In toluene for 0.5h; Ambient temperature; | 97% |
| With water; triethylamine In acetone at 20℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| With N,N-bis[2-oxo-3-oxazolidinyl]phosphorodiamidic chloride; triethylamine In dichloromethane at 20℃; for 0.5h; | 99% |
| With 2,6-dimethylpyridine; tris(2,2'-bipyridyl)ruthenium dichloride; carbon tetrabromide; N,N-dimethyl-formamide at 25 - 30℃; for 12h; Inert atmosphere; Photolysis; | 99% |
| With thionyl chloride In dichloromethane at 22 - 25℃; for 1h; | 98.6% |

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In chloroform at 20℃; for 2h; Acylation; | 98% |
| With 1,4-diaza-bicyclo[2.2.2]octane for 0.1h; | 98% |
-
-
155164-66-2
2-benzoyl-4,5-dichloropyridazin-3(2H)-one

-
A
-
932-22-9
4,5-dichloro-2H-pyridazin-3-one

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In acetonitrile for 7h; Heating; | A n/a B 97% |

| Conditions | Yield |
|---|---|
| Stage #1: benzoic acid With trichloroisocyanuric acid; triphenylphosphine In dichloromethane at 0 - 20℃; Stage #2: Potassium benzoate In dichloromethane at 20℃; for 0.833333h; Reagent/catalyst; Solvent; Time; | 95% |

| Conditions | Yield |
|---|---|
| In toluene at 40℃; for 0.5h; | 92% |
-
-
532-32-1
sodium benzoate

-
-
98-88-4
benzoyl chloride

-
A
-
93-97-0
benzoic acid anhydride

-
B
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| Pyridine 1-oxide hydrochloride In dichloromethane; water at 22℃; Kinetics; Rate constant; Thermodynamic data; mechanism, effect of agitation, influence of ionic strength, other org. solvents, other temperatures; | A 91.8% B n/a |
| In dichloromethane; water Thermodynamic data; influence of ionic strength; |


| Conditions | Yield |
|---|---|
| cobalt(II) chloride In dichloromethane; acetonitrile at 40℃; various acid chlorides, other carboxylic acids; | 91% |
| cobalt(II) chloride In dichloromethane; acetonitrile at 40℃; | 91% |
| With Fe/SWCNTs at 20℃; for 1.41667h; | 90% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In acetonitrile at 85 - 115℃; for 7h; Concentration; Temperature; Large scale; | 91% |
-
-
124-63-0
methanesulfonyl chloride

-
-
65-85-0
benzoic acid

-
A
-
26926-35-2
Benzoesaeure-methansulfonsaeure-anhydrid

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | A 90% B 20% |


| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; water; palladium diacetate; triethylamine In N,N-dimethyl-formamide at 20 - 115℃; under 760.051 Torr; for 6h; Inert atmosphere; Autoclave; | 90% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetrabutyl phosphonium bromide In water; chlorobenzene at 80℃; for 2.5h; Reagent/catalyst; Solvent; Temperature; Sealed tube; | 90% |
| Multi-step reaction with 2 steps 1: trichloroisocyanuric acid / dichloromethane / 20 °C / Inert atmosphere 2: triethylamine / 1 h / 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: trichloroisocyanuric acid / dichloromethane / 20 °C / Inert atmosphere 2: triethylamine; water / dichloromethane / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
22574-12-5
Phenylcarbonyloxy(pyridine-2-thione)

-
A
-
2127-03-9
2,2'-dipyridyldisulphide

-
B
-
127878-49-3
C12H9NO2S

-
C
-
127878-50-6
S-(pyridin-2-yl) pyridine-2-thiosulfonate

-
D
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| In chloroform-d1 at -27℃; for 0.25h; Irradiation; | A 22% B 13% C 20% D 88% |
| In dichloromethane at 0 - 5℃; for 0.5h; Product distribution; Irradiation; other solvent (CDCl3), temperature (-27 deg C), time (5 min); also other acyl derivatives investigated; | A n/a B 11% C n/a D 84% |
| In dichloromethane at -27℃; for 0.0833333h; Irradiation; | A n/a B 21% C n/a D 84% |

-
-
91292-18-1
N,N'carbonyldi<2(3H)-benzoxazolethione>

-
-
65-85-0
benzoic acid

-
A
-
33388-23-7
3-benzoyl-2(3H)-benzoxazolethione

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one In pyridine for 2h; Ambient temperature; | A 88% B n/a |

-
-
10028-70-3
disodium terephthalate

-
-
98-88-4
benzoyl chloride

-
A
-
114833-01-1
C22H14O6

-
B
-
93-97-0
benzoic acid anhydride

-
C
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; Rate constant; | A 88% B 1% C 11% |
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; | A 88% B 1% C 11% |


| Conditions | Yield |
|---|---|
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; Rate constant; | A 12% B 88% |

-
-
57857-69-9
di(p-anisyl)tellurium dibenzoate

-
A
-
4456-36-4
di(p-methoxyphenyl)tellurium dichloride

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With benzoyl chloride In chloroform Heating; | A 87% B 79% |
-
-
57857-69-9
di(p-anisyl)tellurium dibenzoate

-
-
98-88-4
benzoyl chloride

-
A
-
4456-36-4
di(p-methoxyphenyl)tellurium dichloride

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| In chloroform Heating; | A 87% B 79% |

-
-
141-95-7
sodium malonate

-
-
98-88-4
benzoyl chloride

-
A
-
93-97-0
benzoic acid anhydride

-
B
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; Rate constant; | A 13% B 87% |


-
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With water; scandium tris(trifluoromethanesulfonate) In benzene at 120℃; for 18h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Schlenk technique; | 86.9% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In dichloromethane at 0 - 20℃; for 0.333333h; | A 86% B 10% |

-
-
64-17-5
ethanol

-
-
65-85-0
benzoic acid

-
A
-
93-89-0
benzoic acid ethyl ester

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With magnesium chloride at 40℃; for 36h; | A 85% B n/a |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetrabutyl phosphonium bromide In chlorobenzene at 80℃; for 3h; Reagent/catalyst; Solvent; Temperature; Sealed tube; | 85% |
| With tert.-butylhydroperoxide In acetonitrile at 80℃; for 2h; Catalytic behavior; Solvent; Reagent/catalyst; | 82% |
| With tert.-butylhydroperoxide; palladium diacetate In chlorobenzene at 140℃; for 2h; | 80% |
-
-
10027-33-5, 18996-36-6, 25458-19-9
disodium isophthalate

-
-
98-88-4
benzoyl chloride

-
A
-
93-97-0
benzoic acid anhydride

-
B
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; Rate constant; | A 1% B 16% C 83% |
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; | A 1% B 16% C 83% |


-
A
-
93-99-2
benzoic acid phenyl ester

-
B
-
4578-66-9
o-benzoyloxybenzoic acid

-
C
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| In various solvent(s) at 235℃; for 1.33333h; | A 82.1% B 6.3% C 1.5% |

-
-
201024-81-9
C,N-diphenylnitrone

-
A
-
100-52-7
benzaldehyde

-
B
-
93-97-0
benzoic acid anhydride

-
C
-
65-85-0
benzoic acid

-
D
-
17082-12-1
trans-azobenzene

| Conditions | Yield |
|---|---|
| With potassium In tetrahydrofuran for 16h; Product distribution; Mechanism; exclusion of O2, various conditions; | A 42% B 8% C 47% D 82% |


| Conditions | Yield |
|---|---|
| With diphenyl diselenide; dihydrogen peroxide In acetonitrile at 25℃; for 24h; Reagent/catalyst; Temperature; Solvent; Concentration; Green chemistry; | 82% |
-
-
90-05-1
2-methoxy-phenol

-
-
65-85-0
benzoic acid

-
A
-
531-37-3
2-methoxyphenyl benzoate

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In dichloromethane at 0 - 20℃; for 0.333333h; | A 81% B 14% |

-
-
65-85-0
benzoic acid

-
-
927-80-0
1-ethoxyacetylene

-
A
-
38425-59-1
(1-ethoxy)vinyl benzoate

-
B
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In toluene at 40℃; for 15h; | A 80% B 5% |

-
-
17265-13-3
disodium azelainate

-
-
98-88-4
benzoyl chloride

-
A
-
93-97-0
benzoic acid anhydride

-
B
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; Rate constant; | A 3% B 8% C 9% D 80% |
| With Pyridine 1-oxide hydrochloride In dichloromethane; water at 18℃; | A 3% B 8% C 9% D 80% |


| Conditions | Yield |
|---|---|
| With gallium(III) trichloride; silver hexafluoroantimonate In 1,2-dichloro-ethane for 7h; Heating; | 100% |
| With trifluoroacetic acid at 20℃; for 1.5h; Friedel-Crafts Acylation; | 98% |
| With lithium perchlorate In nitromethane at 100℃; for 4h; | 97% |

| Conditions | Yield |
|---|---|
| With 1-[bis(trifluoromethanesulfonyl)methyl]-2,3,4,5,6-pentafluorobenzene | 100% |
| With 4-(1H,1H-perfluorotetradecyl)-C6F4-CH(SO2CF3)2 In toluene at 70℃; for 14h; | 99% |
| With 4-(dimethylamino)pyridine hydrochloride In toluene at 60℃; for 6h; | 98% |
-
-
39687-95-1
isocyanoacetic acid methyl ester

-
-
93-97-0
benzoic acid anhydride

-
-
38061-18-6
methyl 5-phenyl-4-oxazolecarboxylate

| Conditions | Yield |
|---|---|
| With P(MeNCH2CH2)3N In tetrahydrofuran 1.) 5 deg C, 15 min, 2.) r.t., 30 min; | 100% |
| With P(MeNCH2CH2)3N for 2h; | 90% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran |
-
-
90503-12-1
methyl 4,6-O-benzylidene-3-deoxy-3-iodo-α-D-glucopyranoside

-
-
93-97-0
benzoic acid anhydride

-
-
127244-71-7
methyl 2-O-benzoyl-4,6-O-benzylidene-3-deoxy-3-iodo-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 2h; Heating; | 100% |
| With triethylamine; dmap In dichloromethane |
-
-
129090-81-9
methyl (3R)-<3-(2)H>-4-O-benzoyl-6-bromo-3,6-dideoxy-α-D-ribo-hexopyranoside

-
-
93-97-0
benzoic acid anhydride

-
-
131129-47-0
methyl (3R)-<3-(2)H>-2,4-di-O-benzoyl-6-bromo-3,6-dideoxy-α-D-ribo-hexopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 2h; Heating; | 100% |
-
-
99314-92-8
N2-amino-N3-benzyloxycarbonylaminopyridine

-
-
93-97-0
benzoic acid anhydride

-
-
99314-94-0
(2-Benzoylamino-pyridin-3-yl)-carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| With pyridine Ambient temperature; overnight; | 100% |
-
-
100590-12-3
cis-2-(iodomethyl)-3-hydroxytetrahydrofuran

-
-
93-97-0
benzoic acid anhydride

-
-
109788-30-9, 109788-31-0
cis-2-(iodomethyl)-3-benzoxytetrahydrofuran

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran Ambient temperature; | 100% |
-
-
93-97-0
benzoic acid anhydride

-
-
85878-21-3
C12H12F16O4Te

-
-
67103-71-3
1,1,2,2-tetrafluoroethyl benzoate

| Conditions | Yield |
|---|---|
| at 80 - 120℃; | 100% |
-
-
93-97-0
benzoic acid anhydride

-
-
85878-21-3
C12H12F16O4Te

-
A
-
67103-71-3
1,1,2,2-tetrafluoroethyl benzoate

-
B
-
107905-31-7
C20H16F8O6Te

| Conditions | Yield |
|---|---|
| at 20℃; for 24h; Yields of byproduct given; | A n/a B 100% |

-
-
93-97-0
benzoic acid anhydride

-
-
85878-21-3
C12H12F16O4Te

-
A
-
67103-71-3
1,1,2,2-tetrafluoroethyl benzoate

-
B
-
107905-28-2
C16H14F12O5Te

| Conditions | Yield |
|---|---|
| at 20℃; for 2h; ether as solvent;; Yields of byproduct given; | A n/a B 100% |

-
-
93-97-0
benzoic acid anhydride

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane for 2h; | 100% |
-
-
15356-60-2
(1S,2R,5S)-(+)-menthol

-
-
93-97-0
benzoic acid anhydride

-
-
58641-29-5
(2R,5S)-2-isopropyl-5-methyl-cyclohexyl benzoate

| Conditions | Yield |
|---|---|
| erbium(III) triflate In acetonitrile at 50℃; for 1.66667h; | 100% |
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; for 92h; | 96% |
| With scandium tris(trifluoromethanesulfonate) In acetonitrile for 20h; Ambient temperature; | 95% |
| With 2,4,6-tris(2,2-bis(trifluoromethylsulfonyl)ethyl)benzene-1,3,5-triol at 70℃; for 3h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| erbium(III) triflate In acetonitrile at 50℃; for 0.833333h; | 100% |
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile for 0.5h; Heating; | 98% |
| With tris(pentafluorophenyl)borate In neat (no solvent) at 20℃; for 0.166667h; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| With silver perchlorate; niobium pentachloride In nitromethane at 80℃; for 7h; Friedel-Crafts acylation; | 100% |
| With trifluorormethanesulfonic acid; titanium(IV) chloride tris(trifluoromethanesulfonate) In acetonitrile for 12h; Ambient temperature; | 94% |
| Hf[N(SO2C8F17)2]4 In chlorobenzene at 110℃; for 2h; | 94% |
-
-
93-97-0
benzoic acid anhydride

-
-
191924-37-5
2-(trimethylsilyl)ethyl 3-O-benzyl-4,6-O-isopropylidene-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With pyridine for 2h; Ambient temperature; | 100% |
-
-
93-97-0
benzoic acid anhydride

-
-
10212-20-1
2'-deoxy-2'-fluorocytidine

-
-
146954-76-9
N4-benzoyl-2-deoxy-2-fluorocytidine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; | 100% |
| In N,N-dimethyl-formamide at 20℃; | 100% |
| In N,N-dimethyl-formamide | 92% |

| Conditions | Yield |
|---|---|
| ytterbium(III) chloride In tetrahydrofuran Acylation; | 100% |
| With ytterbium(III) chloride In tetrahydrofuran | 100% |
Benzoic anhydride Specification
The Benzoic anhydride is an organic compound with the formula C14H10O3. The IUPAC name of this chemical is benzoyl benzoate. With the CAS registry number 93-97-0, it is also named as phenylcarbonyl benzoate. The product's categories are Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Biochemistry; Nucleosides, Nucleotides & Related Reagents; Protecting Agents for Hydroxyl and Amino Groups; Protecting Agents, Phosphorylating Agents & Condensing Agents; Reagents for Oligosaccharide Synthesis; Carboxylic Acid Anhydrides; Carbonyl Compounds; Organic Building Blocks. Besides, it is a white to almost white crystal or crystalline, which should be stored in a closed cool and dry palce. It is a major industrial chemical widely used for preparing acetate esters, e.g. cellulose acetate.
Physical properties about Benzoic anhydride are: (1)ACD/LogP: 2.738; (2)ACD/LogD (pH 5.5): 2.74; (3)ACD/LogD (pH 7.4): 2.74; (4)ACD/BCF (pH 5.5): 70.94; (5)ACD/BCF (pH 7.4): 70.94; (6)ACD/KOC (pH 5.5): 735.32; (7)ACD/KOC (pH 7.4): 735.32; (8)#H bond acceptors: 3; (9)#Freely Rotating Bonds: 4; (10)Index of Refraction: 1.59; (11)Molar Refractivity: 62.992 cm3; (12)Molar Volume: 186.741 cm3; (13)Polarizability: 24.972 10-24cm3; (14)Surface Tension: 48.5200004577637 dyne/cm; (15)Density: 1.211 g/cm3; (16)Flash Point: 168.44 °C; (17)Enthalpy of Vaporization: 60.566 kJ/mol; (18)Boiling Point: 360 °C at 760 mmHg
Preparation of Benzoic anhydride: Benzoic anhydride is mainly produced by the carbonylation of methyl acetate. Maleic anhydride is produced by the oxidation of benzene or butane. Laboratory routes emphasize the dehydration of the corresponding acids. The conditions vary from acid to acid, but phosphorus pentoxide is a common dehydrating agent:
2 CH3COOH + P4O10 → CH3C(O)OC(O)CH3 + "(HO)2P4O9"
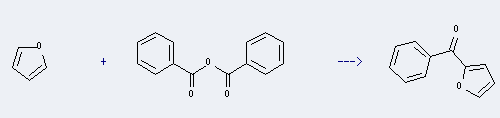
Uses of Benzoic anhydride: it can be used to produce furan-2-yl-phenyl-methanone by heating. This reaction is a kind of Friedel-Crafts acylation. It will need solvent CH2Cl2. The yield is about 80%.
When you are using this chemical, please be cautious about it as the following:
It is risk of serious damage to eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Besides, this chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC(=O)c1ccccc1)c2ccccc2
(2)InChI: InChI=1/C14H10O3/c15-13(11-7-3-1-4-8-11)17-14(16)12-9-5-2-6-10-12/h1-10H
(3)InChIKey: CHIHQLCVLOXUJW-UHFFFAOYAU
(4)Std. InChI: InChI=1S/C14H10O3/c15-13(11-7-3-1-4-8-11)17-14(16)12-9-5-2-6-10-12/h1-10H
(5)Std. InChIKey: CHIHQLCVLOXUJW-UHFFFAOYSA-N
Related Products
- Benzoic 3-chloro-N-ethoxy-2,6-dimethoxybenzimidic anhydride
- Benzoic acid
- Benzoic acid 1-phenyl-2-benzoyl hydrazide
- Benzoic acid 4-hydroxyphenyl ester
- Benzoic acid N,N-diethylamide
- Benzoic acid N-hydroxysuccinimide ester
- Benzoic acid, 2-(2-methylpropoxy)-
- Benzoic acid, 2-(2-phenylethenyl)-, (Z)- (9CI)
- Benzoic acid, 2-(2-thienyl)-
- Benzoic acid, 2-(acetyloxy)-, 3-(hydroxymethyl)phenyl ester
- 939-70-8
- 93972-01-1
- 939757-89-8
- 939759-05-4
- 939759-23-6
- 939759-25-8
- 939791-38-5
- 939791-42-1
- 939792-89-9
- 939793-18-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View