-
Name
Benzopinacole
- EINECS 207-356-8
- CAS No. 464-72-2
- Article Data357
- CAS DataBase
- Density 1.198 g/cm3
- Solubility insoluble in water
- Melting Point 171-173 °C(lit.)
- Formula C26H22O2
- Boiling Point 506.9 °C at 760 mmHg
- Molecular Weight 366.459
- Flash Point 224.9 °C
- Transport Information
- Appearance white crystalline powder
- Safety 22-24/25-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi;
Xi;  Xn
Xn
- Synonyms Benzopinacol(6CI);1,1,2,2-Tetraphenyl-1,2-ethanediol;Benzophenone pinacol;Benzpinacol;Tetraphenyl-1,2-ethanediol;Tetraphenylethylene glycol;a,a'-Bibenzhydrol;
- PSA 40.46000
- LogP 4.85860
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen sulfide; phosphorous acid trimethyl ester In diethylene glycol dimethyl ether for 2.75h; Product distribution; Irradiation; variation of solvents, reagents and conditions; | 100% |
| With hydrogen sulfide; phosphorous acid trimethyl ester In diethylene glycol dimethyl ether for 2.75h; Irradiation; | 100% |
| With iodine; magnesium In diethyl ether; benzene for 0.5h; pinacol coupling; sonication; | 99% |

-
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 100% |

| Conditions | Yield |
|---|---|
| With benzophenone; hydrogen cation In tetrahydrofuran addn. of benzophenone in THF to the Yb-compd., hydrolysis; pptn. of Yb(OH)3, benzpinacol detd. in the THF soln. by LSC; | A 100% B 67% |

| Conditions | Yield |
|---|---|
| With LiCrH4*2LiCl*2THF In tetrahydrofuran at 25℃; for 12h; | A 98% B 2% |
| With triethylamine; cadmium(II) sulphide In methanol for 6h; Irradiation; | A 88% B 11% |
| With sodium tetrahydroborate; nickel dichloride In tetrahydrofuran at 20℃; for 0.25h; | A 88% B 4% |

-
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 98% |
| With hydrogenchloride | 98% |

| Conditions | Yield |
|---|---|
| In benzene for 20h; Product distribution; Quantum yield; Irradiation; | 96% |
-
-
3808-00-2
α-acetoxy-α-phenylbenzeneacetic acid

-
A
-
464-72-2
tetraphenylethane-1,2-diol

-
B
-
108-24-7
acetic anhydride

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile Ambient temperature; electrolysis; | A 93% B n/a |
| With triethylamine In acetonitrile Product distribution; Ambient temperature; electrolysis; | A 93% B n/a |

| Conditions | Yield |
|---|---|
| In benzene for 48h; Irradiation; | 92% |
-
-
626-61-9
4-Chloropyridine

-
-
119-61-9
benzophenone

-
A
-
15031-78-4
2-(4-pyridyl)propan-2-ol

-
B
-
1620-30-0
diphenyl(pyridin-4-yl)methanol

-
C
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol for 24h; Product distribution; Mechanism; Irradiation; conc. H2SO4 added; | A 19% B 6% C 90% |

-
-
119-61-9
benzophenone

-
-
33966-50-6
SEC-BUTYLAMINE

-
-
110-66-7
n-pentanethiol

-
-
75-64-9
tert-butylamine

-
A
-
38836-40-7
N-2-butylidene-2-aminobutane

-
B
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| In benzene for 20h; Product distribution; Quantum yield; Irradiation; | A 76% B 90% |

-
-
119-61-9
benzophenone

-
-
14066-73-0
benzophenone semicarbazone

-
A
-
6344-61-2
9H-fluoren-1-ol

-
B
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| Irradiation; | A 89% B n/a |


| Conditions | Yield |
|---|---|
| With benzophenone semicarbazone Mechanism; Irradiation; other nitrogen-containing reagents; | A 89% B n/a |
| With propan-2-one azine In methanol Irradiation; | A 23% B 1.6 g |

| Conditions | Yield |
|---|---|
| With triethylamine; poly(p-phenylene) In methanol for 6h; Irradiation; | 87% |
-
-
119-61-9
benzophenone

-
-
56859-17-7
(trimethylsilyl)potassium

-
A
-
1450-14-2
1,1,1,2,2,2-hexamethyldisilane

-
B
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane for 12h; Ambient temperature; | A 85% B 80% |

-
-
119-61-9
benzophenone

-
-
15487-82-8, 6735-25-7
triphenylsilylkalium

-
A
-
1450-23-3
hexaphenyldisilane

-
B
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane for 12h; Ambient temperature; | A 79% B 85% |


-
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| samarium(III) chloride In various solvent(s) at 20℃; electrolysis, Al anode; | 85% |
-
-
119-61-9
benzophenone

-
-
124-38-9
carbon dioxide

-
A
-
91-01-0
1,1-Diphenylmethanol

-
B
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| With cadmium(II) sulphide; triethylamine In N,N-dimethyl-formamide at 20℃; for 2h; Irradiation; | A 0% B 85% |
| With cadmium(II) sulphide; triethylamine In N,N-dimethyl-formamide at 20℃; for 2h; Irradiation; | A 78% B 3% |

-
-
119-61-9
benzophenone

-
-
213598-45-9
3,4-isopropylidenedioxy-Δ1-pyrroline-1-oxide

-
A
-
464-72-2
tetraphenylethane-1,2-diol

-
B
-
1384970-76-6
(3aS,4R,6aR)-4-(hydroxydiphenylmethyl)-2,2-dimethyl-tetrahydro-[1,3]dioxolo[4,5-c]pyrrol-5-ol

| Conditions | Yield |
|---|---|
| With samarium diiodide; water In tetrahydrofuran at -78℃; for 2h; Inert atmosphere; diastereoselective reaction; | A 10% B 85% |


| Conditions | Yield |
|---|---|
| With water-d2; magnesium; ethylene dibromide In tetrahydrofuran at 70℃; for 2h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; | A 83% B 12% |
-
-
119-61-9
benzophenone

-
A
-
464-72-2
tetraphenylethane-1,2-diol

-
B
-
7028-47-9
meso-3,4-diphenyladipic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate; water In acetonitrile at -40℃; Irradiation; | A 9% B 8% C 78% |

-
-
119-61-9
benzophenone

-
-
26407-91-0
methyl 2-oxopyrrolidine-1-carboxylate

-
A
-
464-72-2
tetraphenylethane-1,2-diol

-
B
-
1365921-07-8
methyl 2-(diphenylmethylene)pyrrolidine-1-carboxylate

-
C
-
1365921-23-8
C19H21NO4

| Conditions | Yield |
|---|---|
| Stage #1: benzophenone; methyl 2-oxopyrrolidine-1-carboxylate With titanium tetrachloride; zinc In tetrahydrofuran at 0℃; for 12h; McMurry reaction; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 0℃; for 0.25h; | A n/a B 7% C 76% |

-
-
119-61-9
benzophenone

-
-
14092-14-9
4-(methylamino)-3-penten-2-one

-
A
-
464-72-2
tetraphenylethane-1,2-diol

-
B
-
74783-91-8
N-(2,2-diphenyl-2-hydroxyethyl)-4-amino-3-penten-2-one

| Conditions | Yield |
|---|---|
| In benzene for 48h; Irradiation; | A 75% B 17% |

-
-
67-56-1
methanol

-
-
119-61-9
benzophenone

-
A
-
4217-62-3
1,1-diphenyl-1,2-ethanediol

-
B
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| With TiO2 P25 at 20℃; for 48h; UV-irradiation; Inert atmosphere; | A 20% B 71% |


| Conditions | Yield |
|---|---|
| With air; 2,2'-thiobis(2,4-di-tert-butylphenol); triethylamine; copper(l) chloride In tetrahydrofuran at 20℃; for 12h; | 68% |
| With thiobenzoic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; magnesium sulfate; benzaldehyde In N,N-dimethyl acetamide at 20℃; for 24h; Inert atmosphere; Irradiation; | 34% |
| With acetone; butanone; benzene Sonnenlicht; | |
| With benzophenone; benzene Sonnenlicht; | |
| With benzene Sonnenlicht; |
-
-
119-61-9
benzophenone

-
A
-
464-72-2
tetraphenylethane-1,2-diol

-
B
-
7028-47-9
meso-3,4-diphenyladipic acid dimethyl ester

-
C
-
57697-68-4
4,5,5-triphenyldihydrofuran-2(3H)-one

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate; water In acetonitrile for 10h; Product distribution; Mechanism; Ambient temperature; Irradiation; other times, temperature; also under anhydrous conditions in the presence or absence of naphthalene; also for other cyclopropanone acetals and carbonyl compounds; | A 24% B 9% C 3% D 67% |

-
-
100-70-9
2-Cyanopyridine

-
-
119-61-9
benzophenone

-
A
-
19490-90-5
diphenyl(2-pyridyl)methanol

-
B
-
464-72-2
tetraphenylethane-1,2-diol

-
C
-
74439-11-5
[2,4']Bipyridinyl-2'-yl-diphenyl-methanol

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol for 17h; Product distribution; Mechanism; Irradiation; | A 64% B 0.6 g C 2% |
| In water; isopropyl alcohol for 17h; Irradiation; | A 64% B 0.6 g C 2% |

-
-
119-61-9
benzophenone

-
-
762-73-2
trimethyl(allyl)stannane

-
A
-
464-72-2
tetraphenylethane-1,2-diol

-
B
-
4165-79-1
1,1-diphenyl-3-buten-1-ol

| Conditions | Yield |
|---|---|
| In acetonitrile for 5h; Mechanism; Irradiation; other aromatic carbonyl compounds also investigated; | A 10% B 64% |
| In acetonitrile for 5h; Irradiation; | A 10% B 64% |

-
-
22027-65-2
N-[1-morpholino(benzyl)] benzamide

-
-
119-61-9
benzophenone

-
A
-
26581-79-3
3-(diphenylhydroxymethyl)morpholine

-
B
-
464-72-2
tetraphenylethane-1,2-diol

-
C
-
860522-56-1
1,1,2-triphenyl-1-hydroxy-N-benzoylaminoethane

-
D
-
880141-47-9
(+/-)-N,N'-dibenzoyl-1,2-diphenyl-1,2-diaminoethane

| Conditions | Yield |
|---|---|
| In benzene at 30℃; for 4h; Irradiation; uranium filter; | A n/a B n/a C 63% D 5% |

-
-
119-61-9
benzophenone

-
A
-
26581-79-3
3-(diphenylhydroxymethyl)morpholine

-
B
-
464-72-2
tetraphenylethane-1,2-diol

-
C
-
860522-56-1
1,1,2-triphenyl-1-hydroxy-N-benzoylaminoethane

-
D
-
880141-47-9
(+/-)-N,N'-dibenzoyl-1,2-diphenyl-1,2-diaminoethane

| Conditions | Yield |
|---|---|
| With N-[1-morpholino(benzyl)] benzamide In benzene at 30℃; for 4h; Irradiation; uranium filter; | A n/a B n/a C 63% D 5% |

-
-
119-61-9
benzophenone

-
A
-
17530-24-4
di-tert-butylacetylene

-
B
-
464-72-2
tetraphenylethane-1,2-diol

-
C
-
83747-02-8
4,4-Dimethyl-2-pentinsaeure-tert-butylester

-
-
86260-40-4
1,5,6-Tri-tert-butyl-3,3-diphenyl-2-oxabicyclo<2.2.0>hex-5-en-4-carbonsaeure-tert-butylester

| Conditions | Yield |
|---|---|
| With C21H36N2O2 In pentane for 18h; Ambient temperature; Irradiation; | A n/a B 51% C 61% D 7% |


| Conditions | Yield |
|---|---|
| With tellurium tetrachloride In dichloromethane Ambient temperature; | 100% |
| With toluene-4-sulfonic acid solid/solid reaction; | 100% |
| o-benzenedisulfonimide In toluene at 110℃; for 7h; Pinacol rearrangement; | 100% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; triphenylbismuthane; potassium carbonate In water; acetonitrile for 2h; Ambient temperature; | 100% |
| With naphthalene-1,4-dicarbonitrile; oxygen for 90h; Irradiation; | 100% |
| With dinitrogen tetraoxide; ferric nitrate In ethyl acetate Heating; further oxidizing agent; | 100% |
-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
91879-79-7
μ-Oxo-I,I'-bis(trifluoroacetato-O)-I,I'-diphenyldiiodine(III)

-
-
119-61-9
benzophenone

| Conditions | Yield |
|---|---|
| In chloroform for 24h; Product distribution; Ambient temperature; various substrates; | 99% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In benzene for 12h; Ambient temperature; | 99% |
| With trifluorormethanesulfonic acid In benzene for 12h; Mechanism; Ambient temperature; other functionalised pinacols; | 99% |
| With trifluorormethanesulfonic acid In benzene at 10 - 20℃; for 20h; | 75% |
| With trifluorormethanesulfonic acid In toluene at 0 - 20℃; for 24h; Pinacol Rearrangement; Inert atmosphere; | 74% |
| Multi-step reaction with 2 steps 1: SOCl2, Py / CH2Cl2 2: methanol / Irradiation View Scheme |

-
-
464-72-2
tetraphenylethane-1,2-diol

| Conditions | Yield |
|---|---|
| In benzene-d6 for 1 h; | 99% |
-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
1033130-16-3
2-chloro-4,4',5,5'-tetraphenyl-1,3,2-dioxaphospholane

| Conditions | Yield |
|---|---|
| With triethylamine; phosphorus trichloride In tetrahydrofuran at -40 - 20℃; Schlenk technique; | 99% |
| With triethylamine; phosphorus trichloride In tetrahydrofuran at -40 - 20℃; |
-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
78-84-2
isobutyraldehyde

-
A
-
119-61-9
benzophenone

-
B
-
74031-77-9
3-Methyl-1,1-diphenyl-1,2-butanediol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
2043-61-0
cyclohexanecarbaldehyde

-
A
-
119-61-9
benzophenone

-
B
-
20805-08-7
1,1-diphenyl-2-cyclohexylethanediol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
108-94-1
cyclohexanone

-
A
-
119-61-9
benzophenone

-
B
-
15015-46-0
1--1-cyclohexanol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
98-86-2
acetophenone

-
A
-
119-61-9
benzophenone

-
B
-
3784-22-3
1,1,2-triphenyl-1,2-propanediol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
590-86-3
isovaleraldehyde

-
A
-
119-61-9
benzophenone

-
B
-
153595-52-9
4-methyl-1,1-diphenylpentane-1,2-diol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 98% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
100-52-7
benzaldehyde

-
A
-
119-61-9
benzophenone

-
B
-
6296-95-3
1,1,2-triphenyl-1,2-ethanediol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 98% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
104-87-0
4-methyl-benzaldehyde

-
A
-
119-61-9
benzophenone

-
B
-
110247-82-0
1,1-diphenyl-2-p-tolylethane-1,2-diol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 98% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
103-79-7
1-phenyl-acetone

-
A
-
119-61-9
benzophenone

-
B
-
412018-01-0
2-methyl-1,1,3-triphenylpropane-1,2-diol

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 98% |

-
-
464-72-2
tetraphenylethane-1,2-diol

-
-
120-92-3
cyclopentanone

-
A
-
119-61-9
benzophenone

-
B
-
16177-38-1
1-(diphenylhydroxymethyl)-1-hydrohycyclopentane

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 97% |

Benzopinacole Consensus Reports
Reported in EPA TSCA Inventory.
Benzopinacole Specification
The CAS registry number of 1,1,2,2-Tetraphenylethylene glycol is 464-72-2. Its EINECS registry number is 207-356-8. The IUPAC name is 1,1,2,2-tetraphenylethane-1,2-diol. In addition, the molecular formula is C26H22O2 and the molecular weight is 366.45. What's more, it is a kind of white crystalline powder and belongs to the class of Pharmaceutical Intermediates. It is incompatible with strong oxidizing agents. And it should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 5.88; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.88; (4)ACD/LogD (pH 7.4): 5.88; (5)ACD/BCF (pH 5.5): 17300.12; (6)ACD/BCF (pH 7.4): 17300.04; (7)ACD/KOC (pH 5.5): 37599.53; (8)ACD/KOC (pH 7.4): 37599.37; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 7; (12)Polar Surface Area: 18.46 Å2; (13)Index of Refraction: 1.648; (14)Molar Refractivity: 111.29 cm3; (15)Molar Volume: 305.8 cm3; (16)Polarizability: 44.12 ×10-24cm3; (17)Surface Tension: 52 dyne/cm; (18)Density: 1.198 g/cm3; (19)Flash Point: 224.9 °C; (20)Enthalpy of Vaporization: 81.8 kJ/mol; (21)Boiling Point: 506.9 °C at 760 mmHg; (22)Vapour Pressure: 4.26E-11 mmHg at 25°C.
Preparation of 1,1,2,2-Tetraphenylethylene glycol: it can be prepared by benzophenone. This reaction will need reagents Mg and chlorotrimethylsilane, catalyst InCl3 and solvent tetrahydrofuran. The reaction time is 24 hours at reaction temperature of 20 °C. The yield is about 49%.
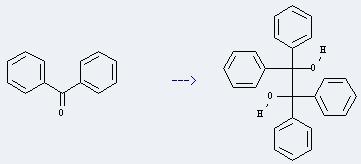
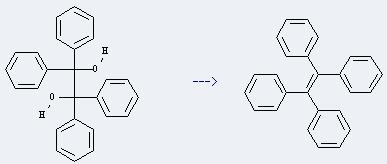
Uses of 1,1,2,2-Tetraphenylethylene glycol: it can be used as organic synthesis intermediates. In addition, it can be used to get tetraphenylethene. This reaction will need reagent titanium slurry and solvent tetrahydrofuran. The reaction time is 20 hours by heating. The yield is about 85%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In addition, you should not breathe dust.
You can still convert the following datas into molecular structure:
(1)SMILES: OC(c1ccccc1)(c2ccccc2)C(O)(c3ccccc3)c4ccccc4
(2)InChI: InChI=1/C26H22O2/c27-25(21-13-5-1-6-14-21,22-15-7-2-8-16-22)26(28,23-17-9-3-10-18-23)24-19-11-4-12-20-24/h1-20,27-28H
(3)InChIKey: MFEWNFVBWPABCX-UHFFFAOYAP
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 8gm/kg (8000mg/kg) | European Journal of Toxicology and Environmental Hygiene. Vol. 9, Pg. 99, 1976. | |
| mouse | LD50 | oral | > 10gm/kg (10000mg/kg) | European Journal of Toxicology and Environmental Hygiene. Vol. 9, Pg. 99, 1976. |
Related Products
- Benzopinacole
- 4647-42-1
- 4647-43-2
- 4648-54-8
- 4649-09-6
- 464916-25-4
- 464917-79-1
- 46492-10-8
- 464-92-6
- 464926-00-9
- 464-98-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View