-
Name
Benzyl benzoate
- EINECS 204-402-9
- CAS No. 120-51-4
- Article Data862
- CAS DataBase
- Density 1.128 g/cm3
- Solubility practically insoluble
- Melting Point 18 °C
- Formula C14H12O2
- Boiling Point 324.1 °C at 760 mmHg
- Molecular Weight 212.248
- Flash Point 147.9 °C
- Transport Information
- Appearance colourless oily liquid with pleasant aromatic odor
- Safety 25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Benzylis benzoas;Peruscabina;Colebenz;Benzyl phenylformate;Benzoesaeurebenzylester;Peruscabin;Antiscabiosum;Ascabiol;Benzyl-benzoate;Benzyl benzoate (natural);Scabide;Ascabin;Benzyl alcohol benzoic ester;Benzyl benzenecarboxylate;Benylate;Vanzoate;Benzylum benzoicum;Benzylbenzenecarboxylate;Phenylmethyl benzoate;Venzonate;Scabagen;Scabanca;benzoic acid, phenylmethyl ester;Benzyl Benzoate , Natural;
- PSA 26.30000
- LogP 3.04360
Synthetic route

| Conditions | Yield |
|---|---|
| With [(ImDippN)Th{N(SiMe3)2}3] In benzene-d6 at 20℃; for 24h; Reagent/catalyst; Time; | 100% |
| With lithium In hexane at 20℃; for 96h; Product distribution; Mechanism; Rate constant; concn. of title compd., reaction time varied; reaction at metal surface; | 99% |
| With bis(1,5-cyclooctadiene)nickel(0); 1,3-bis-(2,6-diisopropylphenyl)-4,5-dichloroimidazol-2-ylidene In toluene at 23 - 60℃; Tishchenko reaction; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With bifunctional polymer In toluene at 20℃; for 6h; | 100% |
| Stage #1: benzoyl chloride; benzyl alcohol In dichloromethane at 20℃; Stage #2: With poly{trans-bicyclo[2.2.1]hept-5-ene-2,3-di(chlorocarbonyl)} In dichloromethane Heating; | 95% |
| With triethylamine | 94% |

| Conditions | Yield |
|---|---|
| With cyanomethylenetributyl-phosphorane In benzene at 100℃; for 24h; | 100% |
| With TiO(acac)2 In xylene for 15h; Heating; | 100% |
| With fluorosulfonyl fluoride; N-ethyl-N,N-diisopropylamine In 1,2-dichloro-ethane at 20℃; for 5h; | 99% |

| Conditions | Yield |
|---|---|
| With 1,3-bis(3,5-bis(trifluoro-ethyl)phenyl)thiourea; 4-pyrrolidin-1-ylpyridine In octane for 24h; Reflux; | 100% |
| With dilithium tetra(tert-butyl)zincate at 0℃; for 1h; Temperature; Inert atmosphere; | 100% |
| With lanthanum(III) isopropoxide; 2-(2-methoxyethoxy)ethyl alcohol In hexane Reflux; chemoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| erbium(III) triflate In acetonitrile at 50℃; for 0.833333h; | 100% |
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile for 0.5h; Heating; | 98% |
| With tris(pentafluorophenyl)borate In neat (no solvent) at 20℃; for 0.166667h; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine at 90℃; for 2h; Reagent/catalyst; Solvent; Ionic liquid; | 100% |
| With triethylamine for 1h; Heating; Inert atmosphere; Ionic liquid; | 99% |
| With caesium carbonate In acetonitrile for 0.5h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| [Cl(C6F13C2H4)2SnOSn(C2H4C6F13)2Cl]2 In toluene at 150℃; for 16h; | 100% |
| With 1,3-bis(2,6-diisopropylphenyl)imidazolylium-2-carboxylate at 120℃; for 3h; Inert atmosphere; | 97% |
| With 1,3-bis(2,6-diisopropylphenyl)imidazolylium-2-carboxylate at 120℃; for 3h; Reagent/catalyst; Concentration; Inert atmosphere; Schlenk technique; | 97% |
| With C16H25N3O2S In n-heptane for 48h; Reflux; Molecular sieve; Inert atmosphere; | 92% |
-
-
10002-30-9
S-pyridin-2-yl benzenecarbothioate

-
-
100-51-6
benzyl alcohol

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| tributylphosphine In dichloromethane at 20℃; | 100% |
-
-
21373-88-6, 16920-54-0
(PPh3)3CoH(N2)

-
-
1579-72-2
2,2,2-trifluoroethyl benzoate

-
A
-
99668-73-2
(trifluoroethoxo)tris(triphenylphosphine)cobalt(I)

-
B
-
120-51-4
benzoic acid benzyl ester

-
C
-
7727-37-9
nitrogen

-
D
-
1333-74-0
hydrogen

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In toluene PhCOOCH2CF3 added to toluene soln. of CoH(N2)(PPh3)3, evacuated, stirred at 20°C for 2 days; | A n/a B 28% C 100% D 17% E 32% |


| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 7h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium fluoride; tetra(n-butyl)ammonium hydrogensulfate In tetrahydrofuran at 20℃; for 3h; | 99% |
| With N-ethyl-N,N-diisopropylamine at 150℃; for 1h; Inert atmosphere; Neat (no solvent); | 99% |
| With cesium fluoride In N,N-dimethyl-formamide at 10 - 15℃; for 24h; | 98% |
-
-
591-50-4
iodobenzene

-
-
201230-82-2
carbon monoxide

-
-
100-51-6
benzyl alcohol

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 100℃; under 5171.62 Torr; for 6h; Autoclave; | 99% |
| With triethylamine at 100℃; under 3750.38 Torr; for 1.5h; Inert atmosphere; | 97% |
| With triethylamine at 100℃; under 3750.38 Torr; for 2h; Autoclave; | 94% |


| Conditions | Yield |
|---|---|
| With C39H31BMnNO2P2; potassium hydride at 150℃; for 24h; | 99% |
| With C42H36ClIrO2P2; caesium carbonate In para-xylene for 36h; Inert atmosphere; Reflux; | 98% |
| With oxygen; vanadia In toluene at 100℃; for 18h; | 97% |

| Conditions | Yield |
|---|---|
| With P(MeNCH2CH2)3N In tetrahydrofuran for 5.5h; Ambient temperature; | 99% |
| With hexamethyltriamido-N-benzylimidophosphate In tetrahydrofuran at 20℃; for 14.5h; Acylation; | 94% |
| With N,N'-bismesityl-imidazol-2-ylidene In tetrahydrofuran at 20℃; for 0.0833333h; | 93% |

-
-
141556-42-5
[1,3-bis(2,4,6-trimethylphenyl)imidazol]-2-ylidene

-
-
100-46-9
benzylamine

-
-
100-51-6
benzyl alcohol

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran-d8 at 60℃; | 99% |


-
-
141556-42-5
[1,3-bis(2,4,6-trimethylphenyl)imidazol]-2-ylidene

-
-
100-51-6
benzyl alcohol

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran-d8 at 20℃; for 24h; | 99% |

-
-
67460-10-0
2-Iodobenzoic acid,benzyl ester

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With Ac-Cys-OMe; bismuth(III) oxide; N-ethyl-N,N-diisopropylamine In dichloromethane at 24℃; Irradiation; | 99% |
| With tetraethylammonium perchlorate; triethylamine In ethanol; dimethyl sulfoxide at 20℃; for 12h; Solvent; Electrolysis; Green chemistry; | 76% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.333333h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With 2,6-dimethyl-1,4-benzoquinone In dichloromethane at 20℃; for 0.5h; Product distribution; Further Variations:; Reagents; | 98% |
| With 2,6-dimethyl-1,4-benzoquinone In dichloromethane at 20℃; for 0.5h; | 98% |
| With 2,6-dimethyl-1,4-benzoquinone In dichloromethane at 20℃; for 1h; | |
| With p-benzoquinone In dichloromethane at 20℃; for 1h; |
-
-
7007-15-0
3-benzoyl-1,3-oxazolidin-2-one

-
-
100-51-6
benzyl alcohol

-
A
-
497-25-6
dimethylenecyclourethane

-
B
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran at 20℃; for 2h; | A n/a B 98% |

-
-
591-50-4
iodobenzene

-
-
100-51-6
benzyl alcohol

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 48h; Reagent/catalyst; Irradiation; Green chemistry; | 98% |
| With C35H20F34NO3(1-)*Pd(2+)*Cl(1-); N-ethyl-N,N-diisopropylamine In neat (no solvent) at 130℃; for 0.333333h; Microwave irradiation; | 96% |
| With potassium phosphate; palladium diacetate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene for 1.5h; Milling; | 91% |
| With tributyl-amine In N,N-dimethyl-formamide at 80℃; for 4h; | 82% |


| Conditions | Yield |
|---|---|
| With zinc(II) oxide In neat (no solvent) at 0 - 20℃; for 2.5h; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid for 24h; Product distribution; Ambient temperature; in CHCl3; | 97% |
| With 3-chloro-benzenecarboperoxoic acid for 24h; Ambient temperature; | 97% |
| With oxygen; benzaldehyde In 1,2-dichloro-ethane at 30℃; for 18h; Baeyer-Villiger Ketone Oxidation; | 65% |

| Conditions | Yield |
|---|---|
| With sodium 4-tert-butylphenolate; sodium t-butanolate In tetrahydrofuran | 97% |
-
-
343227-22-5
Adamantane-1-carboxylic acid cyclohexyl ester

-
-
100-51-6
benzyl alcohol

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With Iron(III) isopropoxide; N-butylpyridinium bis(trifluoromethanesulfonyl)imide; zirconium(IV) tetraisopropoxide In toluene for 7h; | 97% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride at 80℃; for 16h; Retro-Claisen condensation; Neat (no solvent); | 97% |
| With sodium t-butanolate In tert-Amyl alcohol at 120℃; for 18h; Claisen Condensation; Schlenk technique; | 71% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 2h; | 97% |
-
-
74542-54-4
N,N-phenyl-p-toluenesulfonylbenzamide

-
-
100-51-6
benzyl alcohol

-
-
120-51-4
benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile at 100℃; for 15h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Sealed tube; | 97% |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 25℃; for 24h; Reagent/catalyst; Solvent; Temperature; chemoselective reaction; | 100% |
| With hydrogen; palladium diacetate; pyrographite In tetrahydrofuran; methanol at 25℃; under 760.051 Torr; for 12h; | 99% |
| With hydrogen; palladium diacetate; pyrographite In isopropyl alcohol at 25℃; under 760.051 Torr; for 14h; | 99% |

| Conditions | Yield |
|---|---|
| With C56H70Cl3N10Ru2(1+)*F6P(1-); potassium tert-butylate; hydrogen In tetrahydrofuran; dodecane at 70℃; under 37503.8 Torr; for 16h; Inert atmosphere; Glovebox; Autoclave; | 100% |
| With dichloro(benzene)ruthenium(II) dimer; 2-((di-p-tolylphosphino)methyl)-1-methyl-1H-imidazole; potassium tert-butylate; hydrogen In tetrahydrofuran at 100℃; under 37503.8 Torr; for 2h; | 99% |
| With C66H102N4OP2Ru; hydrogen In toluene at 105℃; under 22502.3 Torr; for 20h; Inert atmosphere; Glovebox; | 99% |

| Conditions | Yield |
|---|---|
| With diethylamine; isopropyl alcohol; lithium bromide at 20℃; for 24h; neat (no solvent); chemoselective reaction; | 99% |
| With potassium phosphate; N,N-didecyl-N,N-dimethylammonium bromide at 20℃; for 4.5h; | 97% |
| With Merrifield resin-supported N3=P(MeNCH2CH2)3N at 23 - 25℃; for 5h; Inert atmosphere; | 95% |
| In hexane at 39℃; for 168h; lipase from Pseudomonas fluorescens; | 82.0 % Spectr. |
| With lipase from Pseudomonas fluorescens In hexane at 40℃; for 168h; Product distribution; convenient method for enzymatic benzyl-alkyl transesterification; other benzyl and dibenzyl esters, other aliphatic alcohols, or lipase from Candida cylindracea; influenc of reaction time and and alcohol concentration; |

| Conditions | Yield |
|---|---|
| With trimethylaluminum chloride In toluene for 24h; Heating; | 99% |
-
-
120-51-4
benzoic acid benzyl ester

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
95843-98-4
2-(benzyloxy)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With ToMMgMe at 24.84℃; for 0.5h; | 99% |
| With C12H36MgN2Si4 In neat (no solvent) at 20℃; for 0.166667h; Catalytic behavior; Reagent/catalyst; Temperature; Solvent; Inert atmosphere; Schlenk technique; | 99% |
| With 3C7H21Si3(1-)*La(3+) In benzene at 25℃; for 0.5h; Kinetics; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)-amino]ruthenium(II); potassium tert-butylate In toluene at 120℃; for 48h; Inert atmosphere; Schlenk technique; Reflux; Green chemistry; | 98% |
-
-
120-51-4
benzoic acid benzyl ester

-
-
108-67-8
1,3,5-trimethyl-benzene

-
-
954-16-5
2,4,6-Trimethylbenzophenon

| Conditions | Yield |
|---|---|
| With iodine; phosphorus trichloride In acetonitrile at 160℃; Sealed tube; Schlenk technique; | 98% |
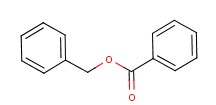
Benzyl benzoate Chemical Properties
Molecular structure of Benzyl benzoate (CAS NO.120-51-4) is:
Product Name: Benzyl benzoate
CAS Registry Number: 120-51-4
IUPAC Name: Benzyl benzoate
Molecular Weight: 212.24388 [g/mol]
Molecular Formula: C14H12O2
XLogP3: 4
H-Bond Donor: 0
H-Bond Acceptor: 2
EINECS: 204-402-9
Melting Point: 18 °C
Surface Tension: 44 dyne/cm
Density: 1.128 g/cm3
Flash Point: 147.9 °C
Enthalpy of Vaporization: 56.61 kJ/mol
Boiling Point: 324.1 °C at 760 mmHg
Vapour Pressure: 0.00025 mmHg at 25°C
Water Solubility: practically insoluble
Stability: Stable. Substances to be avoided include strong oxidizing agents. Combustible.
Benzyl benzoate Uses
Benzyl benzoate (CAS NO.120-51-4) may be used as an antiparasitic insecticide to kill the mites and lice responsible for the skin condition scabies. Also it can be used as a food additive in artificial flavors, a treatment for sweet itch in horses, a solvent for various chemical reactions, a solvent for various chemical reactions, a fixative in fragrances to improve the stability and other characteristics of the main ingredients.
Benzyl benzoate Production
Benzyl benzoate can be generated from benzaldehyde by the Tishchenko reaction.
.jpg)
Benzyl benzoate Safety Profile
Hazard Codes:  Xn
Xn
Risk Statements: 22
R22:Harmful if swallowed.
Safety Statements: 25
S25:Avoid contact with eyes.
WGK Germany: 2
RTECS: DG4200000
HS Code: 29163100
Benzyl benzoate Specification
Benzyl benzoate , its cas register number is 120-51-4. It also can be called Benylate ; Benzoic acid, benzyl ester ; Benzoic acid, phenylmethyl ester ; Phenylmethyl benzoate ; Novoscabin ; Peruscabin ; Peruscabina ; Scabagen ; Scabanca ; Scabide ; Scabiozon ; Scabitox ; Scobenol ; Spasmodin .It is a colourless oily liquid with pleasant aromatic odor.
Related Products
- Benzyl (1-(aminocarbonyl)-2-hydroxypropyl)carbamate
- Benzyl (1-cyano-1-methylethyl)carbamate
- Benzyl (1R,2S)-3-chloro-2-hydroxy-1-(phenylthiomethyl)propylcarbamate
- Benzyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (2R,3S)-(1-carbamoyl-2-hydroxypropyl)carbamate
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3R)-(+)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (3-aminopropyl)carbamate
- Benzyl (3R)-3-aminopiperidine-1-carboxylate
- 1205-17-0
- 120519-47-3
- 120-52-5
- 1205-30-7
- 12053-18-8
- 12053-27-9
- 120-53-6
- 1205-39-6
- 1205-40-9
- 1205-42-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View