-
Name
Bis(trimethylsiloxy)methylsilane
- EINECS 217-496-1
- CAS No. 1873-88-7
- Article Data18
- CAS DataBase
- Density 0.819 g/cm3
- Solubility Miscible with acetone, ethanol, diethyl ether. Insoluble in water.
- Melting Point <0 °C
- Formula C7H22O2Si3
- Boiling Point 163.8 °C at 760 mmHg
- Molecular Weight 222.507
- Flash Point 52.8 °C
- Transport Information UN 1993 3/PG 2
- Appearance Colourless liquid
- Safety 26-36
- Risk Codes 10-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,1,1,3,5,5,5-Heptamethylsiloxane;1,1,1,3,5,5,5-Heptamethyltrisiloxane;2,2,4,6,6-Pentamethyl-3,5-dioxa-2,4,6-trisilaheptane;Bis(trimethylsilyloxy)methylsilane;Heptamethylhydrotrisiloxane;Methylbis(trimethylsiloxy)silane;Methylbis(trimethylsilyloxy)silane;SIB 1844;Di(trimethylsiloxy)methylsilane;
- PSA 18.46000
- LogP 2.53970
Synthetic route
-
-
75-54-7
Dichloromethylsilane

-
-
75-77-4
chloro-trimethyl-silane

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| With Methamphetamin In diethyl ether for 1h; | 68% |
| With water |
-
-
75-54-7
Dichloromethylsilane

-
-
18027-10-6
sodium trimethylsilanolate

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| In diethyl ether for 10h; Heating; | 59% |
-
-
75-54-7
Dichloromethylsilane

-
-
1066-40-6
Trimethylsilanol

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| With pyridine In hexane at -30 - 20℃; for 16h; | 34% |
-
-
99532-16-8
bis(trimethylsiloxy)(trimethylsilyl)methylsilane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
B
-
107-51-7
Octamethyltrisiloxane

| Conditions | Yield |
|---|---|
| at 850℃; | A 14% B 10% |
-
-
75-54-7
Dichloromethylsilane

-
-
107-46-0
Hexamethyldisiloxane

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| In hexane |
-
-
75-54-7
Dichloromethylsilane

-
-
75-77-4
chloro-trimethyl-silane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
B
-
16066-09-4
1,1,1,3,5,7,7,7-octamethyltetrasiloxane

-
C
-
2370-88-9
2,4,6,8-tetramethylcyclotetrasiloxane

-
D
-
17478-13-6
1,1,1,3,5,7,9,9,9-nonamethyl-pentasiloxane

-
E
-
6166-86-5
1,3,5,7,9-pentamethylcyclopentasiloxane

-
F
-
17998-54-8
polymethylhydrosiloxane

| Conditions | Yield |
|---|---|
| With water In toluene at 20℃; for 27h; Product distribution; hydrolytic copolycondensation depending on final concentration of hydrochloric acid; other dialkyldichlorosilanes; | A n/a B 6 % Chromat. C 4 % Chromat. D 3.5 % Chromat. E 3 % Chromat. F 2.0 % Chromat. |

-
-
75-54-7
Dichloromethylsilane

-
-
75-77-4
chloro-trimethyl-silane

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| With Methamphetamin In diethyl ether for 1h; | 68% |
| With water |
-
-
75-54-7
Dichloromethylsilane

-
-
18027-10-6
sodium trimethylsilanolate

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| In diethyl ether for 10h; Heating; | 59% |
-
-
75-54-7
Dichloromethylsilane

-
-
1066-40-6
Trimethylsilanol

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| With pyridine In hexane at -30 - 20℃; for 16h; | 34% |
-
-
99532-16-8
bis(trimethylsiloxy)(trimethylsilyl)methylsilane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
B
-
107-51-7
Octamethyltrisiloxane

| Conditions | Yield |
|---|---|
| at 850℃; | A 14% B 10% |
-
-
75-54-7
Dichloromethylsilane

-
-
107-46-0
Hexamethyldisiloxane

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| In hexane |
-
-
75-54-7
Dichloromethylsilane

-
-
75-77-4
chloro-trimethyl-silane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
B
-
16066-09-4
1,1,1,3,5,7,7,7-octamethyltetrasiloxane

-
C
-
2370-88-9
2,4,6,8-tetramethylcyclotetrasiloxane

-
D
-
17478-13-6
1,1,1,3,5,7,9,9,9-nonamethyl-pentasiloxane

-
E
-
6166-86-5
1,3,5,7,9-pentamethylcyclopentasiloxane

-
F
-
17998-54-8
polymethylhydrosiloxane

| Conditions | Yield |
|---|---|
| With water In toluene at 20℃; for 27h; Product distribution; hydrolytic copolycondensation depending on final concentration of hydrochloric acid; other dialkyldichlorosilanes; | A n/a B 6 % Chromat. C 4 % Chromat. D 3.5 % Chromat. E 3 % Chromat. F 2.0 % Chromat. |

-
-
75-54-7
Dichloromethylsilane

-
-
75-77-4
chloro-trimethyl-silane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
B
-
16066-09-4
1,1,1,3,5,7,7,7-octamethyltetrasiloxane

-
C
-
2370-88-9
2,4,6,8-tetramethylcyclotetrasiloxane

-
D
-
6166-86-5
1,3,5,7,9-pentamethylcyclopentasiloxane

| Conditions | Yield |
|---|---|
| With water In toluene at 20℃; for 27h; Further byproducts given; | A 21 % Chromat. B n/a C 3 % Chromat. D 1.5 % Chromat. |

-
-
1438-82-0
1,1,1,3,3-pentamethyl-1,3-disiloxane

-
A
-
107-46-0
Hexamethyldisiloxane

-
B
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
C
-
107-51-7
Octamethyltrisiloxane

-
D
-
141-62-8
decamethyltetrasiloxane

-
E
-
2895-07-0
1,1,1,3,3,5,5-heptamethyltrisiloxane

-
F
-
77606-50-9
M2DDH

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)carbonyliridium(I) chloride In benzene at 70℃; for 116h; Product distribution; other siloxanes; |

-
-
75-54-7
Dichloromethylsilane

-
-
110-54-3
hexane

-
-
107-46-0
Hexamethyldisiloxane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
B
-
16066-09-4
1,1,1,3,5,7,7,7-octamethyltetrasiloxane

-
C
-
17478-13-6
1,1,1,3,5,7,9,9,9-nonamethyl-pentasiloxane

-
D
-
17998-54-8
polymethylhydrosiloxane

| Conditions | Yield |
|---|---|
| Behandeln des Reaktionsprodukts mit konz.H2SO4; Produkt 5: Tris-trimethylsiloxy-methyl-silan; |


| Conditions | Yield |
|---|---|
| With Lewatit SPC 118 at 90℃; for 4h; |
-
-
75-54-7
Dichloromethylsilane

-
-
107-46-0
Hexamethyldisiloxane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| With hexane; Methamphetamin Behandlung des Reaktionsprodukts mit konz.H2SO4; |

-
-
75-54-7
Dichloromethylsilane

-
-
107-46-0
Hexamethyldisiloxane

-
A
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
B
-
16066-09-4
1,1,1,3,5,7,7,7-octamethyltetrasiloxane

-
C
-
17478-13-6
1,1,1,3,5,7,9,9,9-nonamethyl-pentasiloxane

-
D
-
17998-54-8
polymethylhydrosiloxane

| Conditions | Yield |
|---|---|
| With H2O In hexane; water addn. of (CH3)HSiCl2 to ice, (CH3)3SiOSi(CH3)3 and hexane; stirring of the mixt. with concd. H2SO4 at room temp. for 2 h;; | |
| With water In hexane; water addn. of (CH3)HSiCl2 to ice, (CH3)3SiOSi(CH3)3 and hexane; stirring of the mixt. with concd. H2SO4 at room temp. for 2 h;; | |
| With H2O In hexane; water addn. of (CH3)HSiCl2 to ice, (CH3)3SiOSi(CH3)3 and hexane; stirring of the mixt. with concd. H2SO4 at room temp. for 2 h;; |


| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; for 4h; |
-
-
111-66-0
oct-1-ene

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
17955-88-3
3-n-octyl-1,1,1,3,5,5,5-heptamethyltrisiloxane

| Conditions | Yield |
|---|---|
| With tetradecyl(tributyl)phosphonium bis(trifluoromethylsulfonyl)amide; C24H20Cl2P2Pt at 100℃; for 1h; Reagent/catalyst; | 100% |
| With tetradecyl(tributyl)phosphonium bis(trifluoromethylsulfonyl)amide; bis(cyclooctadiene (μ-silyloxytrimethyl) rhodium(I)) at 110℃; for 1h; Catalytic behavior; Reagent/catalyst; Time; | 100% |
| With nickel 2-ethylhexanoate; 1,4-bis(2,6-diisopropylphenyl)-2,3-dimethyl-1,4-diazabuta-1,3-diene In neat (no solvent) at 23℃; for 6h; regioselective reaction; | 99% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
78827-93-7
Tetraethylene Glycol Mono-2-methylallyl Ether

| Conditions | Yield |
|---|---|
| platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex at 80 - 85℃; Inert atmosphere; Sodium propionate buffer; | 100% |
-
-
592-41-6
1-hexene

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
1873-90-1
3-n-hexyl-1,1,1,3,5,5,5-heptamethyltrisiloxane

| Conditions | Yield |
|---|---|
| With 1,3-bis(tert-buthylethynyl)-1,1,3,3-tetramethyldisiloxane platinum (0) at 50℃; for 16h; Time; Reagent/catalyst; | 100% |
| With C27H55NOPtSi5 In benzene-d6; toluene at 50℃; for 5h; Reagent/catalyst; Concentration; Schlenk technique; Inert atmosphere; | 95% |
| With C24H33N2ORh In tetrahydrofuran at 20℃; for 24h; Time; Inert atmosphere; Glovebox; regioselective reaction; | 86% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
5356-85-4
methylbis(trimethylsilyloxy)vinylsilane

-
-
31576-63-3
C16H44O4Si6

| Conditions | Yield |
|---|---|
| With [Fe(CO)3{(H2C=CHSiMe2O)3SiMe}] at 20℃; for 20h; Catalytic behavior; Reagent/catalyst; Time; Temperature; Concentration; Inert atmosphere; Schlenk technique; | 100% |
| With [Fe(CO)3{(H2C=CHSiMe2)2O}] In decane; toluene at 80℃; for 0.166667h; Reagent/catalyst; Inert atmosphere; | 100% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
536-74-3
phenylacetylene

-
-
198623-99-3
(E)-1,1,1,3,5,5,5-heptamethyl-3-styryltrisiloxane

| Conditions | Yield |
|---|---|
| With C24H23ClCrIrNO3 In 1,1,2,2-tetrachloroethane at 60℃; for 40h; Catalytic behavior; Inert atmosphere; Schlenk technique; regioselective reaction; | 100% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
100342-30-1
thiophene-2-sulfonic acid tert-butylamide

| Conditions | Yield |
|---|---|
| With norborn-2-ene; (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 2.9-dimethyl-1,10-phenanthroline In tetrahydrofuran at 100℃; for 6h; Inert atmosphere; Sealed tube; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3,5,5,5-heptamethyltrisiloxan With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; C25H35N3; cyclohexene In tetrahydrofuran Inert atmosphere; Glovebox; Stage #2: 3-phenylfuran In tetrahydrofuran at 65℃; for 16h; Reagent/catalyst; Sealed tube; Inert atmosphere; regioselective reaction; | 100% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
103628-86-0
8-(allyloxyl)-1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluorooctane

| Conditions | Yield |
|---|---|
| With dichloro(1,5-cyclooctadiene)platinum(ll) In dichloromethane; toluene at 82℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With [Ir(H)(cis-cyclooctene)(CF3SO3)(bis(pyridine-2-yloxy)methylsilyl)]; water In neat (no solvent) at 20℃; Catalytic behavior; | 99% |
| With TiO2 In tert-butyl alcohol Kinetics; 323 K; | |
| With TiO2 In further solvent(s) Kinetics; 323 K, solvent was 2-butoxyethanol; | |
| With TiO2 In isopropyl alcohol Kinetics; 323 K; |
-
-
74-85-1
ethene

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
17861-60-8
3-ethyl-1,1,1,3,5,5,5-heptamethyltrisiloxane

| Conditions | Yield |
|---|---|
| With C22H34FeO2Si4 In toluene at 20℃; for 16h; Inert atmosphere; Schlenk technique; | 99% |
| With C21H34FeO2Si4 In toluene at 20℃; under 760.051 Torr; for 5h; Schlenk technique; | 93% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
A
-
5272-21-9
1,1,1,3,5,5,5-heptamethyltrisiloxan-3-ole

-
B
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| With [Ir(H)(cis-cyclooctene)(CF3SO3)(bis(pyridine-2-yloxy)methylsilyl)]; water In dichloromethane at 20℃; Catalytic behavior; | A 99% B 1 mmol |

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 2,4,7-trimethyl-1,10-phenanthroline In tetrahydrofuran; cyclohexane at 80℃; for 24h; Inert atmosphere; Glovebox; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With cobalt pivalate; 1-adamantanecarbonitrile In neat (no solvent) at 80℃; for 24h; | 99% |
| With C40H72Co2N8 In neat (no solvent) at 50℃; for 24h; | 99% |
| With cobalt pivalate; 1-adamantanecarbonitrile In neat (no solvent) at 80℃; for 3h; Reagent/catalyst; | 99 %Spectr. |
| With (π-Me5C5)Si+B(C6F5)4- In dichloromethane-d2 at 25℃; for 23h; Solvent; Inert atmosphere; | > 98 %Chromat. |
| With chloroplatinic acid 1,1,3,3-tetramethyl-1,3-divinyldisiloxane complex In isopropyl alcohol at 80 - 100℃; for 3h; |

| Conditions | Yield |
|---|---|
| With cobalt pivalate; 1-adamantanecarbonitrile In neat (no solvent) at 80℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 2.9-dimethyl-1,10-phenanthroline In 1,4-dioxane at 100℃; for 6h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Sealed tube; regioselective reaction; | 99% |
| With IrH3{κ3-P,O,P-[9,9-dimethyl-4,5-bis(diisopropylphosphino)xanthene]} In cyclohexene at 110℃; for 18h; Inert atmosphere; Glovebox; | 94% |
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 2,4,7-trimethyl-1,10-phenanthroline In tetrahydrofuran at 120℃; for 40h; Inert atmosphere; Sealed tube; | 66% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
214360-70-0
4,4,5,5-tetramethyl-2-thiophen-3-yl-[1,3,2]dioxaborolane

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3,5,5,5-heptamethyltrisiloxan With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; C25H35N3; cyclohexene In tetrahydrofuran Inert atmosphere; Glovebox; Stage #2: 4,4,5,5-tetramethyl-2-thiophen-3-yl-[1,3,2]dioxaborolane In tetrahydrofuran at 65℃; for 26h; Reagent/catalyst; Sealed tube; Inert atmosphere; regioselective reaction; | 99% |
-
-
292638-84-7
styrene

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
3439-16-5
1,1,1,3,5,5,5-heptamethyl-3-phenethyltrisiloxane

| Conditions | Yield |
|---|---|
| With 1,3-bis(tert-buthylethynyl)-1,1,3,3-tetramethyldisiloxane platinum (0) at 100℃; for 24h; Reagent/catalyst; | 98% |
| With 2,6-bis(1-(2,6-dimethylphenylimino)ethyl)pyridine; bis(cyclooctatetraene)(1-isocyanoadamantane)iron In neat (no solvent) at 80℃; for 23h; Reagent/catalyst; | 92% |
| With bromopentacarbonylmanganese(I) In hexane at 20℃; for 4h; Schlenk technique; Inert atmosphere; Sealed tube; regioselective reaction; | 90% |
-
-
592-76-7
1-Heptene

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
14579-46-5
3-heptyl-1,1,1,3,5,5,5-heptamethyltrisiloxane

| Conditions | Yield |
|---|---|
| With 1,2,3-trimethylimidazolium methylsulphate; bis(cyclooctadiene (μ-silyloxytrimethyl) rhodium(I)) at 90℃; for 2h; Product distribution; Further Variations:; Catalysts; Reagents; | 98% |
| With C77H70N2OPtSi2 In toluene at 80℃; for 24h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; regioselective reaction; | 95% |
| dihydrogen hexachloroplatinate In isopropyl alcohol Heating; | |
| (1,5-cyclooctadiene)rhodium/Aerosil 200 at 100℃; for 1h; | 98 % Chromat. |

| Conditions | Yield |
|---|---|
| With graphene nanoplates-supported platinum nanoparticles In neat (no solvent) at 80℃; for 2h; Catalytic behavior; | 98% |
| With dihydrogen hexachloroplatinate at 75 - 85℃; for 2h; | 96% |
| With dihydrogen hexachloroplatinate at 75 - 85℃; | 96% |

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)-borane In hexane; toluene at 60℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| With [Rh(coe)2OH]2; 3,4,5-MeO-MeO-BIPHEP; cyclohexene In tetrahydrofuran at 45℃; regioselective reaction; | 98% |
-
-
10353-53-4
1,2-epoxy-5-hexene

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| With [Rh(OH)cod]2 and diethylaminopropyltrimethoxysilane immobilized on SiO2 In neat (no solvent) at 40℃; for 2h; | 98% |

| Conditions | Yield |
|---|---|
| With [RuCl(CO)H(PCy3)2(1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene)] In dichloromethane at 20℃; for 1h; Inert atmosphere; Schlenk technique; diastereoselective reaction; | 98% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
53601-12-0
1-vinyl-7-oxabicyclo<4.1.0>heptane

| Conditions | Yield |
|---|---|
| With Pt-GPN (Pt on graphene nanoplatelet) In neat (no solvent) at 60℃; for 2h; | 98% |

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 2.9-dimethyl-1,10-phenanthroline In 1,4-dioxane at 100℃; for 24h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Sealed tube; regioselective reaction; | 98% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
248924-59-6
3-furylboronic acid pinacol ester

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3,5,5,5-heptamethyltrisiloxan With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; C25H35N3; cyclohexene In tetrahydrofuran Inert atmosphere; Glovebox; Stage #2: 3-furylboronic acid pinacol ester In tetrahydrofuran at 80℃; for 20h; Reagent/catalyst; Sealed tube; Inert atmosphere; regioselective reaction; | 98% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 4,4'-di-tert-butyl-2,2'-bipyridine In 1,4-dioxane at 95℃; for 24h; Inert atmosphere; | 98% |
-
-
106-92-3
Allyl glycidyl ether

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
7422-52-8
1,1,1,3,5,5,5-heptamethyl-3-(3-(oxiran-2-ylmethoxy)propyl)trisiloxane

| Conditions | Yield |
|---|---|
| With bis(1-butyl-4-methylpyridinium) hexachloroplatinate(IV) Reagent/catalyst; | 97% |
| With C24H58OPtSi7 In benzene-d6; toluene at 50℃; for 5h; Reagent/catalyst; Schlenk technique; | 95% |
| With cobalt nanoparticles In toluene for 7h; Inert atmosphere; UV-irradiation; Glovebox; Darkness; | 95% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
53326-60-6
2,2,9,9-tetramethyl-3,8-dioxa-2,9-disiladec-5-yne

-
-
128851-10-5
(1,4)-bis(trimethylsiloxy)-3-but-2-ene

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate at 120℃; for 5h; | 97% |
| With dihydrogen hexachloroplatinate In isopropyl alcohol at 125 - 140℃; | |
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex In toluene at 100℃; for 6h; Green chemistry; |
-
-
5390-04-5
pent-1-yn-5-ol

-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
1032732-24-3
(E)-5-(bis(trimethylsilyloxy)(methyl)silyl)-4-penten-1-ol

| Conditions | Yield |
|---|---|
| N-heterocyclic carbene-based platinum(0) complex In toluene at 60℃; for 0.2h; | 97% |
Bis(trimethylsiloxy)methylsilane Specification
The Trisiloxane,1,1,1,3,5,5,5-heptamethyl- with CAS registry number of 1873-88-7 is also known as 1,1,1,3,5,5,5-Heptamethyltrisiloxane. The IUPAC name is Methyl-bis(trimethylsilyloxy)silicon. It belongs to product categories of Industrial/Fine Chemicals; Si (Classes of Silicon Compounds); Siloxanes; Si-O Compounds; Organometallic Reagents; Organosilicon. Its EINECS registry number is 217-496-1. In addition, the formula is C7H22O2Si3 and the molecular weight is 222.51. This chemical is a colourless liquid and should be stored in sealed containers in cool, dry place and away from oxidizing agents.
Physical properties about Trisiloxane,1,1,1,3,5,5,5-heptamethyl- are: (1)ACD/LogP: 5.32; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.32; (4)ACD/LogD (pH 7.4): 5.32; (5)ACD/BCF (pH 5.5): 6517.53; (6)ACD/BCF (pH 7.4): 6517.53; (7)ACD/KOC (pH 5.5): 18694.43; (8)ACD/KOC (pH 7.4): 18694.43; (9)#H bond acceptors: 2; (10)#Freely Rotating Bonds: 4; (11)Flash Point: 52.8 °C; (12)Enthalpy of Vaporization: 38.38 kJ/mol; (13)Boiling Point: 163.8 °C at 760 mmHg; (14)Vapour Pressure: 2.66 mmHg at 25 °C.
Preparation of Trisiloxane,1,1,1,3,5,5,5-heptamethyl-: it is prepared by reaction of dichloro-methyl-silane with chloro-trimethyl-silane. The reaction needs reagent ice and solvent diethyl ether for 1 hour. The yield is about 68%.

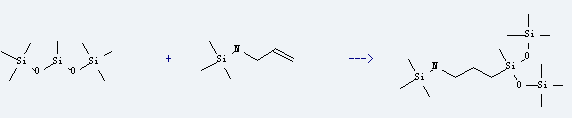
Uses of Trisiloxane,1,1,1,3,5,5,5-heptamethyl-: it can be used as surfactant, and widely used as additive of pesticide and paint. It is used to produce 3-(N-trimethylsilyl-3-aminopropyl)heptamethyltrisiloxane by reaction with allyl-trimethylsilanyl-amine. The reaction occurs with reagent H2PtCl6*6H2O and solvent tetrahydrofuran at 120-145 °C. The yield is about 54%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. Besides, it is flammable. During using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C[Si](O[Si](C)(C)C)O[Si](C)(C)C
2. InChI: InChI=1S/C7H21O2Si3/c1-10(8-11(2,3)4)9-12(5,6)7/h1-7H3
3. InChIKey: SWGZAKPJNWCPRY-UHFFFAOYSA-N
Related Products
- Bis(trimethylsiloxy)methylsilane
- 18738-95-9
- 1873-89-8
- 1873-92-3
- 18739-39-4
- 187396-76-5
- 18740-26-6
- 18740-39-1
- 187404-67-7
- 18741-24-7
- 18741-85-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View