-
Name
TRIPHENYLBOROXIN
- EINECS
- CAS No. 3262-89-3
- Article Data161
- CAS DataBase
- Density 1.13 g/cm3
- Solubility
- Melting Point 217.0 to 221.0 °C
- Formula C18H15B3O3
- Boiling Point 368.3 °C at 760 mmHg
- Molecular Weight 311.748
- Flash Point 176.6 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2,4,6-Triphenylboroxin;Benzeneboronic anhydride;Cyclic benzeneboronic anhydride;Cyclic phenylboronic anhydride;Phenylboronic acid anhydride;Phenylboronic anhydride;Triphenylboroxin;Triphenylboroxole;
- PSA 27.69000
- LogP 1.23600
Synthetic route

| Conditions | Yield |
|---|---|
| at 110℃; for 6h; | 100% |
| at 110℃; for 6h; Inert atmosphere; Neat (no solvent); | 100% |
| Stage #1: phenylboronic acid With barium(II) hydroxide In water at 80℃; for 0.0833333h; Stage #2: With Au38(SCH2CH2Ph)24 In toluene at 80℃; for 12h; Reagent/catalyst; | 100% |
-
-
357417-22-2
N-(4-chlorobenzylidene)-4-methylbenzenesulfonamide

-
-
98-80-6
phenylboronic acid

-
-
3262-89-3
triphenylboroxine

| Conditions | Yield |
|---|---|
| In toluene for 12h; Dean-Stark; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) refluxing equimolar amounts for 48h;; dissolving in CCl4,; | 91% |
| In neat (no solvent) |

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 78℃; for 1h; Catalytic behavior; Reagent/catalyst; Suzuki-Miyaura Coupling; | A n/a B 87% |
| With potassium carbonate In ethanol at 78℃; for 1h; Suzuki-Miyaura Coupling; | A 49% B 6% |


| Conditions | Yield |
|---|---|
| With water; pyridin-2-ylmethyl methanesulphonate In tetrahydrofuran; toluene at 90℃; for 0.666667h; Temperature; Inert atmosphere; | 80.26% |
-
-
7397-50-4
diphenyl(diethylamino)borane

-
-
7360-71-6
N,N'-Bis-(diphenylboryl)-hydrazin

-
-
101550-01-0
(n-C4H9)2BNHNC6H5B(n-C4H9)2

-
-
3262-89-3
triphenylboroxine

| Conditions | Yield |
|---|---|
| byproducts: (C6H5)2CNNC(C6H5)2; 80°C, reflux, 1.5 h; | 75% |
| byproducts: (C6H5)2CNNC(C6H5)2; 80°C, reflux, 1.5 h; | 75% |

-
-
18885-85-3
2-phenyl-4H-benzo-1,3,2-dioxaborine

-
A
-
3262-89-3
triphenylboroxine

-
B
-
100152-08-7
9',9'A-dihydro-4'aH-spiro[chroman-2,1'-xanthen]-2'-one

-
C
-
136352-96-0
C28H24O4

| Conditions | Yield |
|---|---|
| at 900℃; under 0.005 Torr; | A 70% B 12% C 31% |

-
-
110-85-0
piperazine

-
-
98-80-6
phenylboronic acid

-
A
-
3262-89-3
triphenylboroxine

-
B
-
92-54-6
4-phenyl-1-piperazine

-
C
-
92-52-4
biphenyl

| Conditions | Yield |
|---|---|
| With [Cu4I4(1,4-diazabicyclo[2.2.2]octane)2]n In methanol at 27℃; for 5h; | A 9 %Chromat. B 68% C 20 %Chromat. |

-
-
1309575-95-8
2,4-diphenylbenzo[j]-9-methyl-8-aza-1,3,5,2,4-trioxadiboracycloundec-8-ene

-
A
-
3262-89-3
triphenylboroxine

-
B
-
1309575-94-7
(rac)-2-(phenyl)benzo[j]-7-methyl-6-aza-1,3-dioxa-2-boracyclononen-6-ene

| Conditions | Yield |
|---|---|
| In toluene for 24h; Reflux; diastereoselective reaction; | A n/a B 60% |
-
-
1309575-95-8
2,4-diphenylbenzo[j]-9-methyl-8-aza-1,3,5,2,4-trioxadiboracycloundec-8-ene

-
-
98-80-6
phenylboronic acid

-
A
-
3262-89-3
triphenylboroxine

-
B
-
1309575-94-7
(rac)-2-(phenyl)benzo[j]-7-methyl-6-aza-1,3-dioxa-2-boracyclononen-6-ene

| Conditions | Yield |
|---|---|
| In toluene soln. of C6H4C(CH3)N(C2H4OB(C6H5)O)B(C6H5) refluxed for 24 h; solvent and water eliminated, residue washed with CH3Cl, recrystd. from chloroform; elem. anal.; | A n/a B 60% |


| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II); potassium phosphate; oxygen In tetrahydrofuran for 12h; Inert atmosphere; Reflux; | A 60% B 20% C 10% |


| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II); potassium phosphate; oxygen-18 In tetrahydrofuran for 12h; Reflux; | A 33% B 12% C 42% |

-
-
72729-56-7
2,5-diphenyl-1,3,2,5-dioxaborataphosphorinane

-
-
3262-89-3
triphenylboroxine

| Conditions | Yield |
|---|---|
| With methyl iodide In diethyl ether byproducts: bis(hydroxymethyl)methyl(phenyl)phosphonium iodide; heated for 1 h at reflux with protection from atmospheric moisture; solvent removed; residue crystd. (acetone); IR; NMR; elem. anal.; | 40% |
-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
98-80-6
phenylboronic acid

-
A
-
3262-89-3
triphenylboroxine

-
B
-
456-56-4
phenyl trifluoromethylsulfide

-
C
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With potassium fluoride; potassium phosphate; copper(l) iodide; 1,10-Phenanthroline; sulfur; silver carbonate In N,N-dimethyl-formamide at 20℃; Molecular sieve; | A n/a B 33% C n/a |


| Conditions | Yield |
|---|---|
| at 230℃; |

| Conditions | Yield |
|---|---|
| at 175℃; |

| Conditions | Yield |
|---|---|
| With boron trioxide |

| Conditions | Yield |
|---|---|
| Einwirkung von Luftfeuchtigkeit; | |
| Multi-step reaction with 2 steps 1: H2S2 / CS2 2: (hydrolysis) View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether 2: SO2 View Scheme |

| Conditions | Yield |
|---|---|
| Beim Aufbewahren; | |
| room. temp., 1 day; |

| Conditions | Yield |
|---|---|
| With boron trichloride; benzene at 180 - 200℃; ueber mehrere Stufen; |
-
-
17381-62-3
3,5-diphenyl-1,2,4,3,5-trithiaborolane

-
-
3262-89-3
triphenylboroxine

| Conditions | Yield |
|---|---|
| (hydrolysis); |

-
-
3262-89-3
triphenylboroxine

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane Heating; |
-
-
688-74-4
boric acid tributyl ester

-
-
583-53-9
1,2-dibromobenzene

-
A
-
3262-89-3
triphenylboroxine

-
B
-
106-98-9
1-butylene

-
C
-
590-18-1
(Z)-2-Butene

-
D
-
624-64-6
trans-2-Butene


| Conditions | Yield |
|---|---|
| bei der Destillation; | |
| In neat (no solvent) on standing at room temp., reversible react.;; | |
| In neat (no solvent) | |
| In tetrahydrofuran-d8 at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: benzene; boron trichloride / 180 - 200 °C / ueber mehrere Stufen 2: Einwirkung von Luftfeuchtigkeit View Scheme |
-
-
172975-69-8
3,5-dimethylphenyl boronic acid

-
-
98-80-6
phenylboronic acid

-
A
-
3262-89-3
triphenylboroxine

-
D
-
34907-38-5
tris(3.5-dimethyl phenyl)boroxine

| Conditions | Yield |
|---|---|
| With water at 25℃; |


| Conditions | Yield |
|---|---|
| dehydration at 115°C;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) on heating in vac.;; | |
| In neat (no solvent) |
-
-
3262-89-3
triphenylboroxine

-
-
25050-22-0
N-(3,5-dimethylphenyl)-benzamide

| Conditions | Yield |
|---|---|
| With potassium fluoride; chloro(1,5-cyclooctadiene)rhodium(I) dimer In 1,4-dioxane at 100℃; for 24h; Inert atmosphere; Sealed tube; | 100% |
| With potassium fluoride; chloro(1,5-cyclooctadiene)rhodium(I) dimer In 1,4-dioxane at 100℃; for 24h; | 100% |
-
-
3262-89-3
triphenylboroxine

-
-
357417-22-2
N-(4-chlorobenzylidene)-4-methylbenzenesulfonamide

-
A
-
796966-21-7
(R)-N-((4-chlorophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide

-
B
-
796966-17-1
(S)-N-((4-chlorophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With di-μ-chloro-bis[(η5-(4`R,5`R)-(Sp)-2-(2`-4`,5`-dihydro-4`,5`-diphenyl-1`-tosyl-1`H-imidazolyl)cyclopentadienyl,1-C,3`-N)(η5-cyclopentadienyl)-iron(II)]dipalladium(II); silver(I) acetate In chlorobenzene at 65℃; for 20h; Inert atmosphere; | A 100% B 11% |

-
-
3262-89-3
triphenylboroxine

-
-
135822-87-6
(E)-N-(furan-2-ylmethylene)-4-methylbenzenesulfonamide

-
-
158471-23-9
(S)-N-((furan-2-yl(phenyl)methyl)-4-methylbenzenesulfonamide)

| Conditions | Yield |
|---|---|
| With potassium hydroxide; bis(ethylene)rhodium(I) chloride dimer; (1R,4R)-2,5-diphenylbicyclo[2.2.2]octa-2,5-diene In 1,4-dioxane at 60℃; for 6h; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
850661-64-2
(S)-3-methyl-2-[(napthalen-1ylmethyl)-amino]-1,1-diphenyl-butan-1-ol

| Conditions | Yield |
|---|---|
| In toluene microwave irradiation; | 99% |
| With 4 A molecular sieve In toluene for 15h; microwave heating; | |
| In toluene at 200℃; Molecular sieve; Inert atmosphere; Microwave irradiation; |
-
-
3262-89-3
triphenylboroxine

-
-
850661-63-1
(2S)-3-methyl-2-[(octyl)amino]-1,1-diphenyl-butan-1-ol

| Conditions | Yield |
|---|---|
| In toluene microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; bis(1,5-cyclooctadiene)nickel (0) In 1,2-dimethoxyethane; water at 100℃; for 48h; | 99% |
| With bis(1,5-cyclooctadiene)nickel(0); (+/-)-Et-Duphos; sodium t-butanolate In 1,2-dimethoxyethane; water at 100℃; for 48h; | 99% |
| Stage #1: triphenylboroxine With bis(1,5-cyclooctadiene)nickel(0); potassium phosphate; 4-chlorobenzophenone In tetrahydrofuran at 20℃; Stage #2: ortho-anisaldehyde In tetrahydrofuran at 20℃; | 85% |
-
-
3262-89-3
triphenylboroxine

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
134172-65-9, 137474-28-3, 137474-31-8, 13391-45-2
m-methoxybenzhydrol

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; bis(1,5-cyclooctadiene)nickel (0) In 1,2-dimethoxyethane; water at 100℃; for 48h; | 99% |
| With bis(1,5-cyclooctadiene)nickel(0); (+/-)-Et-Duphos; sodium t-butanolate In 1,2-dimethoxyethane; water at 100℃; for 48h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine; (2E,4E)-N,N-diphenyl-2,4-hexadienamide With [IrCl((S,S)-Me-tfb*)]2; potassium carbonate In methanol at 30℃; for 20h; Stage #2: With Crabtree's catalyst; hydrogen In dichloromethane at 20℃; under 760.051 Torr; for 12h; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
1193-18-6
3-methylcyclohexen-2-one

-
-
905832-87-3
(R)-3-methyl-3-phenylcyclohexan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine; 3-methylcyclohexen-2-one With 2C24H14F4*2HO(1-)*2Rh(1+) In 1,4-dioxane at 60℃; for 24h; Inert atmosphere; Stage #2: With water In 1,4-dioxane Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
92553-10-1
3-hydroxy-3-(4-methoxyphenyl)-2,3-dihydro-1H-isoindol-1-one

| Conditions | Yield |
|---|---|
| With C64H46F8Fe4O2Rh2 In 1,4-dioxane at 80℃; for 24h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine With ammonium hexafluorophosphate; palladium(II) trifluoroacetate; 2-[(S)-4,5-dihydro-4-tert-butyl-1,3-oxazol-2-yl]pyridine In 1,2-dichloro-ethane at 20℃; for 0.0333333h; Glovebox; Stage #2: 3-methylcyclohexen-2-one With water-d2 In 1,2-dichloro-ethane for 12h; Glovebox; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
13707-47-6
2-furaldehyde tosylimine

-
-
158471-23-9
(S)-N-((furan-2-yl(phenyl)methyl)-4-methylbenzenesulfonamide)

| Conditions | Yield |
|---|---|
| With chlorobis(ethylene)rhodium(I) dimer; (S)-(-)-[η5-1-bis(3,5-di(trifluormethylphenyl)phosphino-2-(3-diphenylphosphino-2-methylpropenyl)cyclopentadienyl-P)]manganese(I) dicarbonyl; potassium hydroxide In 1,4-dioxane; water at 40℃; for 7h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |
| With chlorobis(ethylene)rhodium(I) dimer; potassium hydroxide; (1R,4R)-2,5-diphenylbicyclo[2.2.2]octa-2,5-diene In 1,4-dioxane; water at 60℃; for 6h; | 97% |
| With chlorobis(ethylene)rhodium(I) dimer; potassium hydroxide; (1R,4R)-2,5-diphenylbicyclo[2.2.2]octa-2,5-diene In 1,4-dioxane; water at 20 - 60℃; for 6.25h; Inert atmosphere; | 97% |
-
-
3262-89-3
triphenylboroxine

-
-
55379-09-4
3-butyl-1,2-benzisothiazole-1,1-dioxide

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine; 3-butyl-1,2-benzisothiazole-1,1-dioxide With chlorobis(cyclooctene)rhodium(I) dimer; C18H23NOS In 1,2-dichloro-ethane at 20℃; for 0.5h; Inert atmosphere; Stage #2: With potassium hydroxide In water; 1,2-dichloro-ethane at 60℃; for 12h; Inert atmosphere; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
131556-56-4
3-benzyl-1,2-benzisothiazole-1,1-dioxide

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine; 3-benzyl-1,2-benzisothiazole-1,1-dioxide With chlorobis(cyclooctene)rhodium(I) dimer; C18H23NOS In 1,2-dichloro-ethane at 20℃; for 0.5h; Inert atmosphere; Stage #2: With potassium hydroxide In water; 1,2-dichloro-ethane at 60℃; for 12h; Inert atmosphere; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine; 3-cyclohexyl-1,2-benziothiazole-1,1-dioxide With chlorobis(cyclooctene)rhodium(I) dimer; C18H23NOS In 1,2-dichloro-ethane at 20℃; for 0.5h; Inert atmosphere; Stage #2: With potassium hydroxide In water; 1,2-dichloro-ethane at 60℃; for 12h; Inert atmosphere; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
34989-82-7
3-methylbenzo[d]isothiazole-1,1-dioxide

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine; 3-methylbenzo[d]isothiazole-1,1-dioxide With chlorobis(cyclooctene)rhodium(I) dimer; C18H23NOS In 1,2-dichloro-ethane at 20℃; for 0.5h; Inert atmosphere; Stage #2: With potassium hydroxide In water; 1,2-dichloro-ethane at 60℃; for 12h; Inert atmosphere; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
357417-22-2
N-(4-chlorobenzylidene)-4-methylbenzenesulfonamide

-
-
796966-21-7
(R)-N-((4-chlorophenyl)(phenyl)methyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With di-μ-chlorobis[η5-(4'R,5'R)-(Sp)-2-(2'-4',5'-dihydro-4',5'-diphenyl-1'-tosyl-1'H-imidazolyl)cyclopentadienyl, (1-C,3'-N)(η5-cyclopentadienyl)-iron(II)]dipalladium(II); silver(I) acetate In chlorobenzene; acetonitrile at 65℃; for 20h; Reagent/catalyst; Temperature; Inert atmosphere; enantioselective reaction; | 99% |
| With di-μ-chloro-bis[(η5-(4`R,5`R)-(Sp)-2-(2`-4`,5`-dihydro-4`,5`-diphenyl-1`-tosyl-1`H-imidazolyl)cyclopentadienyl,1-C,3`-N)(η5-cyclopentadienyl)-iron(II)]dipalladium(II); silver(I) acetate In chlorobenzene at 65℃; for 20h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Schlenk technique; enantioselective reaction; | 99% |
| With chlorobis(ethylene)rhodium(I) dimer; (R)-4-isopropyl-3-((1R,4S)-3-phenylbicyclo[2.2.1]hepta-2,5-diene-2-carbonyl)oxazolidin-2-one; potassium hydroxide; n-octyl β-D-glucopyranoside In 1,4-dioxane; water at 29.84℃; for 1h; Reagent/catalyst; Solvent; Inert atmosphere; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
343598-64-1
N-(2-methylphenyl)methylidene-4-methylbenzenesulfonamide

-
-
738626-20-5
(S)-N-[(2-methylphenyl)phenylmethyl]-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With chlorobis(ethylene)rhodium(I) dimer; potassium hydroxide In tetrahydrofuran at 60℃; for 24h; enantioselective reaction; | 99% |
| With [{(M,SS,SS)-p-Tol-binaso}Rh(OH)]2 In water; toluene at 25℃; for 14h; Glovebox; Sealed tube; Inert atmosphere; enantioselective reaction; | 95% |
-
-
3262-89-3
triphenylboroxine

-
-
160955-96-4
N-[1-(3-Methoxy-phenyl)-meth-(E)-ylidene]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With chlorobis(ethylene)rhodium(I) dimer; potassium hydroxide In tetrahydrofuran at 60℃; for 24h; enantioselective reaction; | 99% |
| With [{(M,SS,SS)-p-Tol-binaso}Rh(OH)]2 In water; toluene at 25℃; for 14h; Glovebox; Sealed tube; Inert atmosphere; enantioselective reaction; | 88% |
-
-
3262-89-3
triphenylboroxine

-
-
14674-38-5
N-(4-methoxybenzylidene)-p-toluenesulfonamide

-
-
258277-16-6, 796966-18-2
(S)-N-((4-methoxyphenyl)(phenyl)methyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With chlorobis(ethylene)rhodium(I) dimer; (S)-(-)-[η5-1-bis(3,5-di(trifluormethylphenyl)phosphino-2-(3-diphenylphosphino-2-methylpropenyl)cyclopentadienyl-P)]manganese(I) dicarbonyl; potassium hydroxide In 1,4-dioxane; water at 40℃; for 7h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |
| With chlorobis(ethylene)rhodium(I) dimer; potassium hydroxide In tetrahydrofuran at 60℃; for 24h; enantioselective reaction; | 29% |
-
-
3262-89-3
triphenylboroxine

-
-
100200-70-2
N-(2-methoxybenzylidene)-p-toluenesulfonamide

-
-
258277-17-7
(S)-N-((2-methoxyphenyl)(phenyl)methyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With chlorobis(ethylene)rhodium(I) dimer; (S)-(-)-[η5-1-bis(3,5-di(trifluormethylphenyl)phosphino-2-(3-diphenylphosphino-2-methylpropenyl)cyclopentadienyl-P)]manganese(I) dicarbonyl; potassium hydroxide In 1,4-dioxane; water at 40℃; for 7h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |
-
-
3262-89-3
triphenylboroxine

-
-
158293-42-6
4-methyl-N-(thiophen-2-ylmethylene)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With chlorobis(ethylene)rhodium(I) dimer; (S)-(-)-[η5-1-bis(3,5-di(trifluormethylphenyl)phosphino-2-(3-diphenylphosphino-2-methylpropenyl)cyclopentadienyl-P)]manganese(I) dicarbonyl; potassium hydroxide In 1,4-dioxane; water at 40℃; for 7h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine; C27H34N2O3SSi With [RhCl(cis-cyclooctene)2]2; (R)-N-(4,4-dimethyl-2-methylenepentyl)-2-methylpropane-2-sulfinamide In toluene at 20℃; for 0.5h; Schlenk technique; Inert atmosphere; Stage #2: With potassium hydroxide In water; toluene at 60℃; for 6h; | 99% |

| Conditions | Yield |
|---|---|
| With [Rh(OH)((S)-binap)]2 at 35℃; for 3h; | 98% |
| With potassium hydroxide; [RhCl((S,S)-2,5-dibenzylbicyclo[2.2.2]octa-2,5-diene)]2 In 1,4-dioxane; water at 30℃; for 1h; | 96% |
-
-
3262-89-3
triphenylboroxine

-
-
100200-70-2
N-(2-methoxyphenyl)methylidene-4-methylbenzenesulfonamide

-
-
258277-17-7
(S)-N-((2-methoxyphenyl)(phenyl)methyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; bis(ethylene)rhodium(I) chloride dimer; (1R,4R)-2,5-diphenylbicyclo[2.2.2]octa-2,5-diene In 1,4-dioxane at 60℃; for 6h; | 98% |

-
-
3262-89-3
triphenylboroxine

-
-
840529-68-2
N-[(S)-(4-methoxyphenyl)(phenyl)methyl]-4-nitrobenzenesulfonamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; [RhCl((1R,5R)-2,6-diphenylbicyclo[3.3.1]nona-2,6-diene)]2 In 1,4-dioxane at 60℃; for 6h; | 98% |
-
-
3262-89-3
triphenylboroxine

-
-
36176-92-8
N-(4-bromophenyl)methylidene-4-nitrobenzenesulfonamide

-
-
840529-67-1
(S)-N-[(4-bromophenyl)phenylmethyl]-4-nitrobenzenesulfonamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; [RhCl((1R,5R)-2,6-diphenylbicyclo[3.3.1]nona-2,6-diene)]2 In 1,4-dioxane at 60℃; for 6h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: triphenylboroxine With diethylzinc In toluene at 60℃; for 4h; Stage #2: 4-fluorobenzaldehyde With (S)-dendritic pyrrolidinylmethanol C116H105NO15 In toluene at -15℃; for 6h; | 98% |
Boroxin,2,4,6-triphenyl- Specification
The Boroxin,2,4,6-triphenyl-, with the CAS registry number 3262-89-3, is also known as Cyclic benzeneboronic anhydride. It belongs to the product category of Organometallics. This chemical's molecular formula is C18H15B3O3 and molecular weight is 311.74. What's more, its IUPAC name is 2,4,6-Triphenyl-1,3,5,2,4,6-trioxatriborinane. It is stable at room temperature and pressure, and it should be stored in a cool and dry place and be protected from light.
Physical properties of Boroxin,2,4,6-triphenyl- are: (1)#H bond acceptors: 3; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 3; (4)Polar Surface Area: 27.69 Å2; (5)Index of Refraction: 1.564; (6)Molar Refractivity: 89.77 cm3; (7)Molar Volume: 275.6 cm3; (8)Polarizability: 35.58×10-24 cm3; (9)Surface Tension: 38.1 dyne/cm; (10)Density: 1.13 g/cm3; (11)Flash Point: 176.6 °C; (12)Enthalpy of Vaporization: 59.08 kJ/mol; (13)Boiling Point: 368.3 °C at 760 mmHg; (14)Vapour Pressure: 2.72E-05 mmHg at 25 °C.
Preparation: this chemical can be prepared by 2-phenyl-4H-benzo[1,3,2]dioxaborinine at the temperature of 900 °C. The yield is about 12%.
![Boroxin,2,4,6-triphenyl- can be prepared by 2-phenyl-4H-benzo[1,3,2]dioxaborinine at the temperature of 900 °C.](/UserFilesUpload/Preparation of Boroxin,2,4,6-triphenyl(2).jpg)
Uses of Boroxin,2,4,6-triphenyl-: it can be used to produce 4-nitro-biphenyl at the temperature of 65 °C. It will need reagent K2CO3 and solvents acetone, H2O with the reaction time of 45 min. This reaction will also need catalyst palladium acetate. The yield is about 97%.
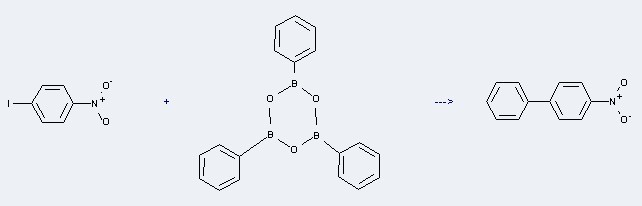
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: B1(OB(OB(O1)C2=CC=CC=C2)C3=CC=CC=C3)C4=CC=CC=C4
(2)InChI: InChI=1S/C18H15B3O3/c1-4-10-16(11-5-1)19-22-20(17-12-6-2-7-13-17)24-21(23-19)18-14-8-3-9-15-18/h1-15H
(3)InChIKey: VOXXGUAZBWSUSS-UHFFFAOYSA-N
Related Products
- Boroxin,2,4,6-triphenyl-
- Boroxin,2,4,6-tris(4-methylphenyl)-
- 32631-29-1
- 32634-66-5
- 32634-68-7
- 32634-95-0
- 32638-88-3
- 3264-10-6
- 3264-21-9
- 3264-38-8
- 32644-12-5
- 32644-15-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View