-
Name
Bromoform
- EINECS 200-854-6
- CAS No. 75-25-2
- Article Data209
- CAS DataBase
- Density 2.997 g/cm3
- Solubility 3.2 g/L at 30 °C in water
- Melting Point 8 °C
- Formula CHBr3
- Boiling Point 149.2 °C at 760 mmHg
- Molecular Weight 252.731
- Flash Point 44 °C
- Transport Information UN 2515 6.1/PG 3
- Appearance colourless to yellow liquid
- Safety 28-45-61-28A-36/37-16-7
- Risk Codes 23-36/38-51/53-22-39/23/24/25-23/24/25-11
-
Molecular Structure
-
Hazard Symbols
 T,
T,  N,
N,  F
F
- Synonyms Methenyl tribromide;NSC 8019;Tribromomethane;Tribromomethane;Methyl tribromide;Tribrommethan;NCI-C55130;CCRIS 98;
- PSA 0.00000
- LogP 2.45470
Synthetic route
-
-
941-98-0
1'-naphthacetophenone

-
A
-
75-25-2
Bromoform

-
B
-
2459-24-7
methyl 1-naphthoate

-
C
-
149-73-5
trimethyl orthoformate

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A n/a B 88% C n/a |

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
A
-
75-25-2
Bromoform

-
B
-
121-98-2
methyl 4-methoxybenzoate

-
C
-
149-73-5
trimethyl orthoformate

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A n/a B 85% C n/a |

-
-
281-23-2
adamantane

-
-
75-05-8
acetonitrile

-
A
-
768-90-1
1-Adamantyl bromide

-
B
-
75-25-2
Bromoform

-
C
-
23074-42-2
1-adamantanecarbonitrile

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; molybdenum hexacarbonyl at 140 - 160℃; for 6h; Reactivity; Mechanism; Reagent/catalyst; | A 15% B n/a C 85% |


| Conditions | Yield |
|---|---|
| With 2+ In water; acetonitrile at 20℃; Mechanism; Rate constant; Product distribution; | A 4% B 84% |
| With Co(II)W12O407-; sodium perchlorate In water; acetonitrile at 20℃; for 2h; Rate constant; Product distribution; pH 7; reaction in MeCN/D2O; reaction between CBr4 and the heteropoly blues Co(II)W12O407- and Co(II)W12O408-, kinetic and product stidy, effect of inert salts,; | A 1 % Spectr. B 77 % Spectr. |
-
-
122-00-9
para-methylacetophenone

-
A
-
99-75-2
4-methyl-benzoic acid methyl ester

-
B
-
75-25-2
Bromoform

-
C
-
149-73-5
trimethyl orthoformate

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A 83% B n/a C n/a |

-
-
98-86-2
acetophenone

-
A
-
93-58-3
benzoic acid methyl ester

-
B
-
75-25-2
Bromoform

-
C
-
149-73-5
trimethyl orthoformate

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide at 30℃; for 4h; Product distribution; Mechanism; electrolysis (Pt-anode, Cu-Zn (60:40) cathode, 220 mA/cm2 constant current); other electrolyte, other electrolyte-to-substrate ratio, other material of electrodes; | A 74% B n/a C 15% |
| With methanol; sodium bromide at 30℃; for 4h; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A 74% B n/a C n/a |

-
-
592-41-6
1-hexene

-
-
558-13-4
carbon tetrabromide

-
A
-
75-25-2
Bromoform

-
B
-
3740-44-1
1,1,1,3-tetrabromoheptane

-
C
-
90278-16-3
1,1,3-tribromoheptane

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) at 80℃; for 4h; Product distribution; further reagent, further conditions; | A 3.7% B 70% C 10.1% D 9.1% |
| With 2,2'-azobis(isobutyronitrile) at 80℃; for 4h; | A 3.7% B 70% C 10.1% D 9.1% |

-
-
29949-72-2
di(tert-butyl)(n-butyl)phosphine

-
A
-
75-25-2
Bromoform

-
B
-
81193-50-2
butyl-di-t-butylchlorophosphonium bromide

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; hydrogen chloride In diethyl ether 1) -120 deg C to -50 deg C 2) 0 deg C, Et2O; | A 70% B 70% |
-
-
563-80-4
3-methyl-butan-2-one

-
A
-
75-25-2
Bromoform

-
B
-
547-63-7
Methyl isobutyrate

-
C
-
149-73-5
trimethyl orthoformate

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A n/a B 67% C n/a |

-
-
106053-65-0
4,5-Dimethoxy-2-(2-phenylethyl)acetophenon

-
A
-
75-25-2
Bromoform

-
B
-
106053-67-2
2-Brom-4,5-dimethoxybibenzyl

-
C
-
106053-50-3
4,5-Dimethoxy-2-(2-phenylethyl)benzoesaeure

| Conditions | Yield |
|---|---|
| With sodium hydroxide; bromine In 1,4-dioxane for 1h; Ambient temperature; | A n/a B 2% C 65% |
| With sodium hydroxide; bromine In 1,4-dioxane for 1h; Ambient temperature; | A n/a B 2% C 65% |

-
-
111-13-7
hexyl-methyl-ketone

-
A
-
75-25-2
Bromoform

-
B
-
19841-72-6
2,2-dimethoxy-3-octanol

-
C
-
106-73-0
methyl heptanoate

-
D
-
149-73-5
trimethyl orthoformate

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A n/a B 29% C 62% D n/a |
| With methanol; sodium bromide at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A n/a B 29% C 62% D n/a |

-
-
122-57-6
1-Phenylbut-1-en-3-one

-
A
-
75-25-2
Bromoform

-
B
-
103-26-4
methyl cinnamate

-
C
-
149-73-5
trimethyl orthoformate

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | A n/a B 61% C n/a |

-
-
34557-54-5
methane

-
A
-
74-83-9
methyl bromide

-
B
-
75-25-2
Bromoform

-
C
-
558-13-4
carbon tetrabromide

-
D
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With bromine at 500℃; Product distribution; Further Variations:; Temperatures; Bromination; | A 17.4% B 0.48% C 2.5% D 5% |

-
-
56-23-5
tetrachloromethane

-
-
108565-60-2
N-(2,2,2-tribromo-1-hydroxy-ethyl)-butyramide

-
A
-
541-35-5
butanamide

-
B
-
75-25-2
Bromoform


| Conditions | Yield |
|---|---|
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| With sodium hypobromide |

| Conditions | Yield |
|---|---|
| With aluminium trichloride |


| Conditions | Yield |
|---|---|
| With bromine at 400℃; |
-
-
64-17-5
ethanol

-
-
558-13-4
carbon tetrabromide

-
A
-
74-96-4
ethyl bromide

-
B
-
75-25-2
Bromoform

-
C
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| at 150℃; |


| Conditions | Yield |
|---|---|
| With calcium bromide | |
| With calcium bromide Electrolysis; | |
| With potassium bromide Electrolysis; | |
| With potassium bromide Electrolysis; | |
| With calcium bromide Electrolysis; |

| Conditions | Yield |
|---|---|
| With bromine |

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide | |
| With aluminum tri-bromide | |
| With aluminum tri-bromide; hydrogen bromide |

| Conditions | Yield |
|---|---|
| With alkaline arsenite | |
| With alkaline sulfite | |
| With phenylhydrazine |

| Conditions | Yield |
|---|---|
| With sodium hypobromide |

| Conditions | Yield |
|---|---|
| With bromine |

| Conditions | Yield |
|---|---|
| With ammonia; water |

| Conditions | Yield |
|---|---|
| With sodium at 30℃; for 4h; | 100% |
-
-
1195-32-0
1-methyl-4-isopropenylbenzene

-
-
75-25-2
Bromoform

-
-
87959-45-3
1-(2,2-dibromo-1-methylcyclopropyl)-4-methylbenzene

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In dichloromethane; water at 40 - 45℃; for 22h; Inert atmosphere; | 100% |
| With sodium hydroxide In dichloromethane at 40℃; | 73% |
| With potassium tert-butylate In hexane at 0℃; for 2h; | 50% |
-
-
75-25-2
Bromoform

-
-
83466-54-0, 93602-74-5, 79073-99-7
Bis(2,4,6-tri-tert-butylphenyl)diphosphen

-
-
111888-02-9
1,2-bis(2,4,6-tri-tert-butylphenyl)3,3-dibromo diphosphirane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In hexane at 15℃; for 2h; sonication; | 100% |
| With n-butyllithium In diethyl ether at 0℃; | |
| With potassium tert-butylate CBr4 + n-BuLi is also possible; |

| Conditions | Yield |
|---|---|
| With methanol; sodium at 30℃; electrolysis (Pt-anode, Cu-Zn (60:40)-cathode, 220 mA/cm2 constant current density); | 100% |
| With methanol; sodium at 30℃; for 4h; Product distribution; electrolysis (Pt-anode, Cu-Zn (60:40) cathode, 220 mA/cm2 constant current); reaction with NaBr/CH3OH; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: Bromoform With lithium diisopropyl amide In tetrahydrofuran; hexane at -100℃; for 0.166667h; Metallation; Stage #2: 4-heptanone With boron trifluoride diethyl etherate In tetrahydrofuran; diethyl ether; hexane at -90℃; for 4h; Addition; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: Bromoform With lithium diisopropyl amide In tetrahydrofuran; hexane at -100℃; for 0.166667h; Metallation; Stage #2: cycloactanone With boron trifluoride diethyl etherate In tetrahydrofuran; diethyl ether; hexane at -90℃; for 4h; Addition; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: Bromoform With lithium diisopropyl amide In tetrahydrofuran; hexane at -100℃; for 0.166667h; Metallation; Stage #2: 1-phenyl-propan-1-one With boron trifluoride diethyl etherate In tetrahydrofuran; diethyl ether; hexane at -90℃; for 4h; Addition; | 100% |
-
-
75-25-2
Bromoform


| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In diethyl ether at 25℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether under Ar; addn. CHBr3/ether to soln. W-complex/ether, soln. kept 2 h (crystn.); solvent decanted, solid washed (ether), dried (vac.), elem. anal.; | 100% |
-
-
75-25-2
Bromoform

-
-
1055313-43-3
C26H56O5Si3

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; | 100% |
-
-
67-56-1
methanol

-
-
75-25-2
Bromoform

-
-
179056-82-7
4-(5-methyl-1,3,4-oxadiazol-2-yl)benzaldehyde

-
-
1333472-39-1
2-methoxy-2-(4-(5-methyl-1,3,4-oxadiazol-2-yl)phenyl)acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: methanol; Bromoform; 4-(5-methyl-1,3,4-oxadiazol-2-yl)benzaldehyde With potassium hydroxide In 1,4-dioxane at 20℃; Cooling with ice; Stage #2: With hydrogenchloride In water pH=1; | 100% |


| Conditions | Yield |
|---|---|
| With benzyltriethylammonium bromide; sodium hydroxide In water at 0 - 20℃; for 2h; | 100% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 0 - 60℃; | 80% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 50 - 60℃; for 2h; Inert atmosphere; | |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 50 - 60℃; Inert atmosphere; | |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; |
-
-
156042-18-1, 81741-00-6, 145842-96-2
trans-(η(5)-pentamethylcyclopentadienyl)(dicarbonyl)(dihydrido)rhenium

-
-
75-25-2
Bromoform

| Conditions | Yield |
|---|---|
| Heating; Schlenk technique; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: (η5:η5-4,4',5,5'-tetra-tert-butylfulvalene)[Ir(CO)2]2; benzene for 8h; Irradiation; Inert atmosphere; Stage #2: Bromoform at 50℃; for 12h; | A 100% B 100% |


| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; sodium hydroxide In dichloromethane; water at 5℃; | 99% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; Inert atmosphere; | 80% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; Inert atmosphere; | 80% |
-
-
75-25-2
Bromoform

-
-
62322-46-7
3-(1-methoxy-1-methylethoxy)prop-1-ene

-
-
259194-62-2
1,1-Dibromo-2-[(1-methoxy-1-methylethoxy)methyl]cyclopropan

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; triethylamine In dichloromethane at 20℃; for 48h; cyclocondensation; | 99% |
-
-
75-25-2
Bromoform

-
-
63922-74-7
(E)-1-(Trimethylsilyl)-2-nonene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In hexane at 0 - 20℃; | 99% |
-
-
75-25-2
Bromoform

-
-
40595-34-4
(E)-trimethyl(3-phenylallyl)silane

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In hexane at 0 - 20℃; | 99% |
-
-
75-25-2
Bromoform

-
-
15870-10-7
2-methylhept-1-ene

-
-
197661-72-6
1,1-dibromo-2-methyl-2-pentyl-cyclopropane

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In dichloromethane; water at 40 - 45℃; for 22h; Inert atmosphere; | 99% |
| With sodium hydroxide; tetrabutylammomium bromide In water at 55℃; for 1h; |
-
-
75-25-2
Bromoform

-
-
1712-70-5
1-chloro-4-(1-methylethenyl)-benzene

-
-
1064000-89-0
1-chloro-4-(2,2-dibromo-1-methylcyclopropyl)benzene

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; Inert atmosphere; | 99% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; Inert atmosphere; | 99% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In dichloromethane; water at 40 - 45℃; for 22h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In water | 99% |
-
-
75-25-2
Bromoform

-
-
611-15-4
1-methyl-2-vinyl-benzene

-
-
132608-14-1
1-(2,2-dibromocyclopropyl)-2-methylbenzene

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; Inert atmosphere; | 99% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 50 - 60℃; for 2h; | 41% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 50 - 60℃; for 2h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: 4-(tert-butyldiphenylsilyloxy)hepta-1,6-diene With Grubbs catalyst first generation In dichloromethane at 20℃; for 0.333333h; Inert atmosphere; Stage #2: Bromoform With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In dichloromethane for 6h; Inert atmosphere; Sonication; | 99% |
-
-
75-25-2
Bromoform

-
-
57204-39-4
4-(vinyl-β,β-d2)-1,1'-biphenyl

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; Inert atmosphere; | 99% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 20 - 60℃; Inert atmosphere; | 99% |
-
-
563-79-1
2,3-Dimethyl-2-butene

-
-
75-25-2
Bromoform

-
-
22715-57-7
2,2,3,3-tetramethyl-1,1-dibromocyclopropane

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In n-heptane at -9 - 20℃; for 4h; | 98% |
| With potassium tert-butylate In pentane | 95% |
| With potassium tert-butylate In n-heptane at -10 - 20℃; Inert atmosphere; Industrial scale; | 76% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In ethanol; dichloromethane for 5h; | 98% |
| With potassium tert-butylate In tert-butyl alcohol; pentane at 0℃; for 14h; | 93% |
| With potassium tert-butylate at -25℃; | 91% |
-
-
75-25-2
Bromoform

-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
-
62405-61-2
2,2,2',2'-tetrabromo-1,1'-dimethyl-1,1'-bicyclopropyl

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane for 1.5h; ultrasound treatment; | 98% |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride 1.) 30 min, 0 deg C, 2.) 2 d, r.t.; | 63% |
| With sodium hydroxide | 35% |
| With potassium tert-butylate In pentane |
-
-
75-25-2
Bromoform

-
-
38898-42-9
3-methylenetetracyclo<3.2.0.02,7,04,6>heptane

-
-
81787-72-6
2,2-Dibromspiro2,7.04,6>heptan>

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride at 40℃; for 4h; | 98% |
| With potassium tert-butylate In hexane at 0℃; for 0.75h; | 72% |
-
-
75-25-2
Bromoform

-
-
3446-89-7
4-(Methylthio)benzaldehyde

-
-
109086-16-0
2-hydroxy-2-(4-methylthiophenyl)ethanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; lithium bromide In 1,4-dioxane | 98% |
| With potassium hydroxide; lithium bromide In 1,4-dioxane; water 1.) 0 deg C, 18 h, 2.) 23 deg C, 24 h; | 7.6 g |

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 1h; Ambient temperature; | 98% |
Bromoform Consensus Reports
Bromoform Standards and Recommendations
ACGIH TLV: TWA 0.5 ppm (skin); Animal Carcinogen
DFG MAK: Confirmed Animal Carcinogen with Unknown Relevance to Humans
DOT Classification: 6.1; Label: KEEP AWAY FROM FOOD
Bromoform Analytical Methods
Bromoform Specification
The Bromoform with CAS registry number of 75-25-2 is also known as Methane, tribromo-. The IUPAC name and product name are the same. It belongs to product categories of Pharmaceutical Intermediates; Organics; Heterocyclic Compounds; Analytical Chemistry; Solvents for HPLC & Spectrophotometry; Solvents for Spectrophotometry; Standard Solution of Volatile Organic Compounds for Water & Soil Analysis; Standard Solutions (VOC); 600 Series Wastewater Methods; A-BAlphabetic; Alpha Sort; B; BI - BZChemical Class; BromoEPA; Halogenated; Method 601; Volatiles/ Semivolatiles. Its EINECS registry number is 200-854-6. In addition, the formula is CHBr3 and the molecular weight is 252.73.
Physical properties about Bromoform are: (1)ACD/LogP: 2.29; (2)ACD/LogD (pH 5.5): 2.29; (3)ACD/LogD (pH 7.4): 2.29; (4)ACD/BCF (pH 5.5): 32.39; (5)ACD/BCF (pH 7.4): 32.39; (6)ACD/KOC (pH 5.5): 419.56; (7)ACD/KOC (pH 7.4): 419.56; (8)Index of Refraction: 1.619; (9)Molar Refractivity: 29.83 cm3; (10)Molar Volume: 84.9 cm3; (11)Surface Tension: 49.8 dyne/cm; (12)Density: 2.974 g/cm3; (13)Flash Point: 44 °C; (14)Enthalpy of Vaporization: 39.66 kJ/mol; (15)Boiling Point: 149.2 °C at 760 mmHg; (16)Vapour Pressure: 5.17 mmHg at 25 °C.
Preparation of Bromoform: it is prepared by reaction of acetone with sodium hypobromite under alkaline conditions. Then product is derived after decomposition, distillation, washing, filtration and drying.
![]()
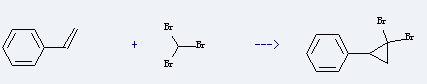
Uses of Bromoform: it is used to produce 1,1-dibromo-2-phenyl-cyclopropane by reaction with vinylbenzene. The reaction occurs with reagent potassium tert-butoxide and solvent pentane at ambient temperature for 10 hours. The yield is about 65%. This cehmical is also can be used as dye intermediate, disinfectant, analgesic, anesthetic, refrigerant, mineral agent, precipitating agent, solvent and liquid component of blast-resistant.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes and skin. It's toxic to aquatic organisms and may cause long-term adverse effects in the aquatic environment. What's more, it has danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing and gloves. Avoid release to the environment. In case of accident or if you feel unwell seek medical advice immediately. After using it, keep container tightly closed and away from sources of ignition. After contact with skin, wash immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C(Br)(Br)Br
2. InChI: InChI=1S/CHBr3/c2-1(3)4/h1H
3. InChIKey: DIKBFYAXUHHXCS-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | LDLo | oral | 143mg/kg (143mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 141, 1969. | |
| mammal (species unspecified) | LC50 | inhalation | 12100mg/m3 (12100mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 18(4), Pg. 55, 1974. | |
| mouse | LD50 | intraperitoneal | 1274mg/kg (1274mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 20(12), Pg. 52, 1976. | |
| mouse | LD50 | oral | 1072mg/kg (1072mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | National Toxicology Program Technical Report Series. Vol. NTP-TR-350, Pg. 1989, |
| mouse | LD50 | subcutaneous | 1820mg/kg (1820mg/kg) | Toxicology and Applied Pharmacology. Vol. 4, Pg. 354, 1962. | |
| rabbit | LDLo | subcutaneous | 410mg/kg (410mg/kg) | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 28, Pg. 201, 1891. | |
| rat | LCLo | inhalation | 45gm/m3/4H (45000mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 28, 1982. | |
| rat | LD50 | intraperitoneal | 414mg/kg (414mg/kg) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE BEHAVIORAL: ANTIPSYCHOTIC BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Toxicology Letters. Vol. 15, Pg. 251, 1983. |
| rat | LD50 | oral | 933mg/kg (933mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | National Toxicology Program Technical Report Series. Vol. NTP-TR-350, Pg. 1989, |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View