-
Name
Carbaryl
- EINECS 200-555-0
- CAS No. 63-25-2
- Article Data46
- CAS DataBase
- Density 1.183 g/cm3
- Solubility 0.00826 g/100 mL in water
- Melting Point 142-146 °C(lit.)
- Formula C12H11NO2
- Boiling Point 329.3 °C at 760 mmHg
- Molecular Weight 201.225
- Flash Point 153 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance colourless solid
- Safety 22-24-36/37-46-61-2-62-60
- Risk Codes 22-40-50-67-65-50/53-38-11
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  N,
N,  F
F
- Synonyms Karbaryl;Atoxan;Dicarbament 23,969;Carbatox 75;1-Naftylester kyseliny methylkarbaminove [Czech];N-Methyl-alpha-naphthylurethan;Murvin;Hexavin;Karbaryl [Polish];Suleo;ENT-23969;XLR-Plus;Denapon;N-Methyl-alpha-naphthylcarbamate;Menaphtam;alpha-Naphthalenyl methylcarbamate;Karbaspray;EPA Pesticide Chemical Code 056801;Experimental Insecticide 7744;Methylcarbamate 1-naphthol;N-Metil-1-naftil-carbammato [Italian];1-Naphthalenol, methylcarbamate;Cekubaryl;ENT 23,969;Carbatox-75;Sewin;N-Methyl-1-naftyl-carbamaat;N-Methylcarbamate de 1-naphtyle [French];Vioxan;Monsur;Carylderm;Latka 7744 [Czech];Carbamic acid, methyl-, 1-naphthyl ester;Dicarbam;Bug master;Carbaril [Italian];N-Methyl-.alpha.-naphthylurethan;Carbamine;UC 7744;1-Naphthyl-N-methyl-karbamat [German];N-Metil-1-naftil-carbammato;Vetox;Tricarnam;.alpha.-Naftyl-N-methylkarbamat;Thinsec;1-Naphthalenol, methylcarbamate (9CI);Methylcarbamate 1-naphthalenol;Carbatox-60;Panam;Caprolin;1-Naphthalenol methylcarbamate;Toxan;Septene;Sevin;Carbavur;Carbaril [INN];Laivin;Seffein;N-Methyl-1-naphthyl carbamate;Suvamil;
- PSA 38.33000
- LogP 2.94890
Synthetic route

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 60℃; for 7h; Mechanism; analogous reaction of other carbamates; | 88% |
| With trichlorophosphate at 60℃; for 7h; | 88% |
-
-
22013-97-4
N-methyl-thiocarbamic acid S-methyl ester

-
-
90-15-3
α-naphthol

-
-
63-25-2
1-naphthalenol methylcarbamate

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 4h; Reflux; | 80% |
| With zinc(II) chloride In benzene for 21h; Heating; | 50% |
-
-
90-15-3
α-naphthol

-
-
124-38-9
carbon dioxide

-
-
74-89-5
methylamine

-
-
63-25-2
1-naphthalenol methylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide; methylamine With N-ethyl-N,N-diisopropylamine In dichloromethane at -40℃; for 0.75h; Stage #2: α-naphthol With trichlorophosphate In dichloromethane Stage #3: With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane | 80% |

-
-
132905-86-3
Carbonic acid chloromethyl ester naphthalen-1-yl ester

-
-
74-89-5
methylamine

-
-
63-25-2
1-naphthalenol methylcarbamate

| Conditions | Yield |
|---|---|
| In hexane; water for 2h; Ambient temperature; | 75.4% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 18h; Reagent/catalyst; Time; Inert atmosphere; | 65% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; 1,10-Phenanthroline; copper diacetate In decane at 20℃; for 2h; Molecular sieve; Inert atmosphere; | 45% |

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 60℃; for 8h; | 33% |

| Conditions | Yield |
|---|---|
| With pyridine; benzene | |
| In Dimethyldisulphide at 20℃; for 0.166667h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / Et3N / CH2Cl2 / 1.67 h / 5 °C 2: 75.4 percent / H2O; hexane / 2 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| With Isopropylbenzene; methanesulfonic acid In water; toluene | |
| With Isopropylbenzene; methanesulfonic acid In water; toluene |

| Conditions | Yield |
|---|---|
| With trichlorophosphate In water; toluene | |
| With trichlorophosphate In water; toluene | |
| With phosphorus tribromide In 1,1-dichloroethane |

| Conditions | Yield |
|---|---|
| With methylamine In toluene |
-
-
50-00-0
formaldehyd

-
-
63-25-2
1-naphthalenol methylcarbamate

-
-
39073-17-1
Chloromethyl-methyl-carbamic acid naphthalen-1-yl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride |

| Conditions | Yield |
|---|---|
| aluminium In water; acetonitrile Product distribution; Irradiation; influence of aluminum foil;; |

| Conditions | Yield |
|---|---|
| With water; aluminum oxide In methanol at 57℃; Mechanism; other temperature, flow velocity; |

| Conditions | Yield |
|---|---|
| With (5,10,15,20-tetrakis(pentafluorophenyl)porphyrinato)iron(III) chloride; dihydrogen peroxide for 0.5h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With rabbit serum albumin at 37℃; for 5h; pH=7.4; Enzyme kinetics; | |
| With bovin serum albumin Kinetics; aq. buffer; Enzymatic reaction; | |
| With potassium hydroxide at 20℃; under 760.051 Torr; Electrochemical reaction; Inert atmosphere; | |
| With sodium hydroxide In water at 80℃; for 10h; |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iron(II); sodium chloride at 24.7℃; Product distribution; Activation energy; Further Variations:; Temperatures; var. ratio of reagents; Electrochemical reaction; | |
| With dihydrogen peroxide; iron; sodium chloride In water at 25℃; Activation energy; Product distribution; Further Variations:; Temperatures; Electrolysis; |

-
-
63-25-2
1-naphthalenol methylcarbamate

-
-
67271-09-4
N,N'-bis-(dibutylamino)disulfide

-
-
67316-53-4
1-naphthyl (dibutylaminothio)methylcarbamate

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; triethylamine In hexane; water |
-
-
63-25-2
1-naphthalenol methylcarbamate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / water 2: hydrogenchloride; sodium nitrite / water View Scheme |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water |
-
-
624-92-0
Dimethyldisulphide

-
-
63-25-2
1-naphthalenol methylcarbamate

-
-
137-41-7
potassium N-methyldithiocarbamate

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; acetonitrile for 0.0111111h; Time; Microwave irradiation; |

| Conditions | Yield |
|---|---|
| In ethanol |

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol |

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol |
Carbaryl Chemical Properties
IUPAC Name: naphthalen-1-yl N-methylcarbamate
Empirical Formula: C12H11NO2
Molecular Weight: 201.2212g/mol
EINECS: 200-555-0
Structure of Carbaril (CAS NO.63-25-2):
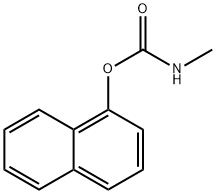
Index of Refraction: 1.611
Molar Refractivity: 59.03 cm3
Molar Volume: 169.9 cm3
Polarizability: 23.4×10-24cm3
Surface Tension: 45.1 dyne/cm
Density: 1.183 g/cm3
Flash Point: 153 °C
Enthalpy of Vaporization: 57.18 kJ/mol
Melting point: 142 ºC
Boiling Point: 329.3 °C at 760 mmHg
Vapour Pressure: 0.000179 mmHg at 25°C
Water solubility: Insoluble. 0.00826 g/100 mL
Product Categories: Nitrogen Compounds;Organic Building Blocks;Protected Amines;AcaricidesAlphabetic;CA - CGPesticides;CarbamatesMethod Specific;Endocrine Disruptors (Draft)Method Specific;C;EPA;Insecticides;Oeko-Tex Standard 100;Pesticides;Pesticides&Metabolites;AcaricidesPesticides;PesticidesAlphabetic;SA - SM;S;AcaricidesPesticides&Metabolites;Alpha sort;CAlphabetic;PesticidesPesticides&Metabolites;Plant growth regulators
Canonical SMILES: CNC(=O)OC1=CC=CC2=CC=CC=C21
InChI: InChI=1S/C12H11NO2/c1-13-12(14)15-11-8-4-6-9-5-2-3-7-10(9)11/h2-8H,1H3,(H,13,14)
InChIKey: CVXBEEMKQHEXEN-UHFFFAOYSA-N
Carbaryl History
Carbaryl is a colorless white crystalline solid commonly sold under the brand name Sevin, a trademark of the Bayer Company. Union Carbide discovered carbaryl and introduced it commercially in 1958. Bayer purchased Aventis CropScience in 2002, a company that included Union Carbide pesticide operations.
Carbaryl Uses
Carbaryl is used chiefly as an insecticide.
Carbaryl Toxicity Data With Reference
| 1. | skn-rbt 12 mg/24H SEV | JAFCAU Journal of Agricultural and Food Chemistry. 9 (1961),30. | ||
| 2. | eye-rbt 500 mg/24H MOD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,164. | ||
| 3. | mmo-sat 250 µg/plate | RPZHAW Roczniki Panstwowego Zakladu Higieny. 30 (1979),81. | ||
| 4. | mma-hmn:fbr 1 µmol/L | MUREAV Mutation Research. 42 (1977),161. | ||
| 5. | dns-hmn:fbr 1 µmol/L | MUREAV Mutation Research. 42 (1977),161. | ||
| 6. | cyt-hmn:emb 40 µg/kg | ZDVKAP Zdravookhranenie. Public Health. 20 (4)(1977),14. | ||
| 7. | orl-man TDLo:500 mg/kg:PNS | NEURAI Neurology. 37 (1987),1229. | ||
| 8. | orl-rat LD50:230 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 11 (1967),546. | ||
| 9. | skn-rat LD50:4000 mg/kg | 85DPAN Wirksubstanzen der Pflanzenschutz und Schadlingsbekampfungsmittel Werner Perkow,Berlin, Germany.: Verlag Paul Parey,1971/76. | ||
| 10. | ipr-rat LD50:64 mg/kg | PSEBAA |
Carbaryl Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 , 1987,p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 12 , 1976,p. 37.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Community Right-To-Know List.
Carbaryl Safety Profile
Hazard Codes:  Xn,
Xn, N,
N, F
F
Risk Statements: 22-40-50-67-65-50/53-38-11
R22:Harmful if swallowed.
R40:Limited evidence of a carcinogenic effect.
R50:Very toxic to aquatic organisms.
R67:Vapours may cause drowsiness and dizziness.
R65:Harmful: may cause lung damage if swallowed.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R38:Irritating to skin.
R11:Highly flammable. .
Safety Statements: 22-24-36/37-46-61-2-62-60
S22:Do not breathe dust.
S24:Avoid contact with skin.
S36/37:Wear suitable protective clothing and gloves.
S46:If swallowed, seek medical advice immediately and show this container or label.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
S2:Keep out of the reach of children.
S62:If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label.
S60:This material and its container must be disposed of as hazardous waste.
RIDADR: UN 2811 6.1/PG 3
WGK Germany: 3
RTECS: FC5950000
HazardClass: 6.1(b)
PackingGroup: III
Poison by ingestion, intravenous, intraperitoneal, and possibly other routes. Human systemic effects by ingestion: sensory change involving peripheral nerves and muscle weakness. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Human mutation data reported. An eye and severe skin irritant. Absorbed by all routes, although skin absorption is slow. No accumulation in tissue. Symptoms include blurred vision, headache, stomachache, vomiting. Symptoms similar to but less severe than those due to parathion. A reversible cholinesterase inhibitor. See also CARBAMATES and ESTERS. When heated to decomposition it emits toxic fumes of NOx.
Carbaryl Standards and Recommendations
OSHA PEL: TWA 5 mg/m3
ACGIH TLV: TWA 5 mg/m3; Not Classifiable as a Human Carcinogen
DFG MAK: 5 mg/m3
NIOSH REL: (Carbaryl) TWA 5 mg/m3
Carbaryl Analytical Methods
For occupational chemical analysis use OSHA: #ID-63 or NIOSH: Carbaryl, 5006.
Carbaryl Specification
Carbaril , its cas register number is 63-25-2. It also can be called 1-Naphthalenol methylcarbamate ; 1-Naphthol methylcarbamate ; 1-Naphthyl N-methylcarbamate ; Arylam ; Dicarbam ; Bug Master ; Carbamec ; Carbamine ; Carbatox ; Carbomate ; Carpolin ; Carylderm ; Cekubaryl ; Clinicide ; Denapon ; Derbac ; Devicarb ; Hexavin ; Karbaspray ; Murvin ; NAC ; OMS 29, UC 7744 ; Padrin ; Panam ; Patrin ; Ravyon ; Savit ; Seffein ; Septene ; Sevimol ; Sevin ; Tercyl; Thinsec ; Tornado ; Tricarnam . Carbaril (CAS NO.63-25-2) is a colourless solid.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View