-
Name
Clavulanic acid
- EINECS 261-069-2
- CAS No. 58001-44-8
- Article Data18
- CAS DataBase
- Density 1.65 g/cm3
- Solubility Soluble in water
- Melting Point
- Formula C8H9NO5
- Boiling Point 545.8 °C at 760 mmHg
- Molecular Weight 199.163
- Flash Point 283.9 °C
- Transport Information
- Appearance Colorless needle-like crystal
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (Z)-(2R,5R)-3-(2-Hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo(3.2.0)heptane-2-carboxylic acid;Acide clavulanique;Acidum clavulanicum;Antibiotic MM 14151;BRL 14151;BRN 0787059;Clavulansaeure;MM 14151;UNII-23521W1S24;
- PSA 87.07000
- LogP -1.15770
Synthetic route
-
-
57943-84-7
Benzyl Clavulanate

-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol under 760 Torr; for 0.75h; Ambient temperature; | 84% |
-
-
103545-38-6, 103664-39-7, 103664-40-0, 135909-75-0, 135909-76-1
(2RS,5S)-<5-(3)H>Ornithine

-
B
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| With cultures of Streptomyces clavuligerus (ATCC 27064) in a glycerol-based fermentation medium Product distribution; labelling studies also with incorporation (14)C, acid isolated as p-bromobenzyl ester; |

| Conditions | Yield |
|---|---|
| With cultures of Streptomyces clavuligerus (ATCC 27064) in a glycerol-based fermentation medium Product distribution; labelling studies also with incorporation (14)C, acid isolated as p-bromobenzyl ester; |

-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| Streptomyces clavuligerus SC 2; |

| Conditions | Yield |
|---|---|
| Streptomyces clavuligerus; examination of incorporation by labelled <5-(14)C, 5-(3)H>ornithine; |
-
-
57943-82-5
methyl clavulanate

-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 70 percent / sodium hydroxide / H2O / 1.17 h / 0 °C 2: 69 percent / dimethylformamide / 1.5 h / Ambient temperature 3: 84 percent / H2 / palladium on carbon / ethanol / 0.75 h / 760 Torr / Ambient temperature View Scheme |
-
-
57943-81-4
potassium clavulanate

-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 69 percent / dimethylformamide / 1.5 h / Ambient temperature 2: 84 percent / H2 / palladium on carbon / ethanol / 0.75 h / 760 Torr / Ambient temperature View Scheme |

-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| With NADPH at 26℃; pH=7.0; |
-
-
4013-94-9
N,N-diisopropyl-1,2-ethanediamine

-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| In methanol; water; ethyl acetate for 0.25h; | 96% |
| In ethyl acetate; isopropyl alcohol | 84% |
| In methanol; ethyl acetate | 81% |

| Conditions | Yield |
|---|---|
| In ethyl acetate | 83% |
| In ethanol; ethyl acetate for 1h; | 68% |
| In ethyl acetate; isopropyl alcohol | 67% |
-
-
768-94-5
1-Adamantanamine

-
-
58001-44-8
clavulanic acid

-
-
66069-33-8
(2R,5R)-3-[2-Hydroxy-eth-(Z)-ylidene]-7-oxo-4-oxa-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid; compound with adamantan-1-ylamine

| Conditions | Yield |
|---|---|
| In ethyl acetate | 81% |
| In ethanol; ethyl acetate | 80% |
| In methanol; ethyl acetate for 1h; | 76% |
| In ethyl acetate; isopropyl alcohol | 76% |

| Conditions | Yield |
|---|---|
| In ethyl acetate | 81% |
| In ethyl acetate; isopropyl alcohol for 1h; | 77% |
| In ethanol; ethyl acetate | 43% |
| Stage #1: clavulanic acid With ammonium sulfate In water; ethyl acetate at 5℃; pH=1.30; Stage #2: tert-butylamine In methanol; ethyl acetate at 5℃; for 2h; pH-value; Concentration; |
-
-
107-45-9
tert-Octylamine

-
-
58001-44-8
clavulanic acid

-
-
66069-32-7
(2R,5R)-3-[2-Hydroxy-eth-(Z)-ylidene]-7-oxo-4-oxa-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid; compound with 1,1,3,3-tetramethyl-butylamine

| Conditions | Yield |
|---|---|
| In ethyl acetate | 80% |
| In ethyl acetate; isopropyl alcohol for 1h; | 67% |
| In ethanol; ethyl acetate | 35% |
-
-
58001-44-8
clavulanic acid

-
-
83670-77-3
methyl-4-aza-8-hydroxy-6-oxo-oct-2-enoate

| Conditions | Yield |
|---|---|
| With methanol; diethylamine for 0.166667h; Ambient temperature; | 78% |

| Conditions | Yield |
|---|---|
| In ethanol Ambient temperature; | 75% |
-
-
58001-44-8
clavulanic acid

-
-
477947-66-3
9-chloro-9-deoxyclavulanic acid

| Conditions | Yield |
|---|---|
| With pyridine; methanesulfonyl chloride In acetonitrile at 25℃; for 24h; | 75% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dicyclohexyl-carbodiimide In tetrahydrofuran; dichloromethane; ethyl acetate | 67% |

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate for 0.5h; | 61% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Ambient temperature; | 60% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In acetonitrile Ambient temperature; | 59% |

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate for 1h; | 54% |
| In ethyl acetate; isopropyl alcohol | 31% |
| In ethyl acetate | 3% |

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate for 1h; | 52.6% |
| In ethyl acetate; isopropyl alcohol | 52% |
| In ethyl acetate | 8% |
-
-
103-74-2
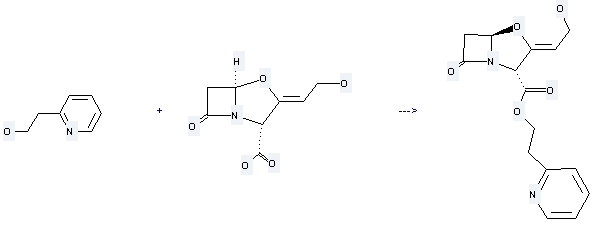
2-(2-Hydroxyethyl)pyridine

-
-
58001-44-8
clavulanic acid

-
-
91022-06-9
2-(2-pyridyl)ethyl clavulanate

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In acetonitrile Ambient temperature; | 50% |
-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| In ethyl acetate; isopropyl alcohol for 1h; | 37% |
| In ethyl acetate | 30% |
-
-
58001-44-8
clavulanic acid

-
A
-
58243-07-5
methyl (E)-3-(diethylamino)-2-propenoate

-
B
-
83670-73-9
methyl 3-(2-hydroxyethyl)-pyrrole-4-carboxylate

-
C
-
83670-77-3
methyl-4-aza-8-hydroxy-6-oxo-oct-2-enoate

| Conditions | Yield |
|---|---|
| With diethylamine In methanol for 48h; Ambient temperature; | A 20% B 14% C 18% |
| With Diethyl amine In methanol for 48h; Ambient temperature; | A 20% B 14% C 18% |


| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In acetonitrile Ambient temperature; | 17% |
-
-
58001-44-8
clavulanic acid

| Conditions | Yield |
|---|---|
| With mercury(II) diacetate In 1,2-dimethoxyethane 1) r.t., 15 min, 2) 90-95 deg C (bath), 20 min; | A 5% B 5.5% |
-
-
58001-44-8
clavulanic acid

-
-
71019-00-6
(5R)-7-oxo-3-vinyl-4-oxa-1-azabicyclo[3.2.0]hept-2-ene

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide dimethyl acetal In tetrahydrofuran | |
| With N,N-dimethyl-formamide dimethyl acetal In tetrahydrofuran for 0.133333h; Ambient temperature; Yield given; | |
| With N,N-dimethyl-formamide dimethyl acetal |
-
-
501-53-1
benzyl chloroformate

-
-
58001-44-8
clavulanic acid

-
-
92632-80-9
1-benzyloxycarbonylamino-4-hydroxybutan-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; sodium hydrogencarbonate 1.) 2 h, 2.) water, THF, ice-cooling, 40 min; Yield given. Multistep reaction; |
-
-
937-14-4
3-chloro-benzenecarboperoxoic acid

-
-
58001-44-8
clavulanic acid

-
-
71916-37-5
(Z)-(3S,5R)-2-(2-hydroxyethylidene)-3-(m-chlorobenzoyloxy)clavam

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In 1,2-dimethoxyethane; dichloromethane 1.) 0 deg C, 3 h, 2.) room temperature, 15 h; | 60 mg |
-
-
67-56-1
methanol

-
-
58001-44-8
clavulanic acid

-
A
-
57943-82-5
methyl clavulanate

-
B
-
88367-33-3
(Z)-(2R)-5-(2-hydroxyethylidene)-2-methoxycarbonylmethyl-2,5-dihydro-oxazole

-
C
-
88391-46-2
(Z)-(3S,5R)-2-(2-hydroxyethylidene)-3-methoxyclavam

| Conditions | Yield |
|---|---|
| With triethylamine at -30℃; for 0.833333h; electrolysis: platinum electrodes, I=200-250 mA; | A 5 mg B 75 mg C 10 mg |
| With triethylamine at -30℃; for 0.833333h; electrolysis: platinum electrodes, current 200-250 mA; | A 5 mg B 75 mg C 10 mg |

-
-
67-56-1
methanol

-
-
58001-44-8
clavulanic acid

-
A
-
57943-82-5
methyl clavulanate

-
B
-
88391-46-2
(Z)-(3S,5R)-2-(2-hydroxyethylidene)-3-methoxyclavam

-
C
-
71916-39-7, 88391-48-4, 88391-49-5
(E)-(3S,5R)-3-acetoxy-2-formylmethyleneclavam

-
D
-
88391-49-5
(Z)-(3S,5R)-3-acetoxy-2-formylmethyleneclavam

| Conditions | Yield |
|---|---|
| With triethylamine at -30℃; for 0.833333h; Product distribution; electrolysis, platinum electrodes, current 200-250 mA; reactions of oxidative decarboxylation ( electrolysis, with Pb(OAc)4, decarboxylative rearrangement of diacyl peroxide) of clavulanic acid and its methyl ether; | A 5 mg B 10 mg C n/a D n/a |

-
-
546-67-8
lead(IV) tetraacetate

-
-
58001-44-8
clavulanic acid

-
A
-
88391-50-8
(Z)-(3S,5R)-3-acetoxy-2-(2-hydroxyethylidene)clavam

-
B
-
88391-47-3
(Z)-(3S,5R)-3-acetoxy-2-(2-acetoxyethylidene)clavam

-
C
-
71916-39-7, 88391-48-4, 88391-49-5
(E)-(3S,5R)-3-acetoxy-2-formylmethyleneclavam

-
D
-
88391-49-5
(Z)-(3S,5R)-3-acetoxy-2-formylmethyleneclavam

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane; benzene at 70℃; for 0.333333h; | A 145 mg B 20 mg C n/a D n/a |
| In 1,2-dimethoxyethane; benzene at 70℃; for 0.333333h; Yields of byproduct given; | A 145 mg B 20 mg C n/a D n/a |
| In 1,2-dimethoxyethane; benzene at 70℃; for 0.333333h; Yield given. Title compound not separated from byproducts; | A 145 mg B 20 mg C n/a D n/a |

Clavulanic acid History
Clavulanic acid was discovered around 1974/75 by British scientists working at the drug company Beecham. After several attempts, Beecham finally filed for US patent protection for the drug in 1981, and U.S. Patents 4,525,352, 4,529,720 and 4,560,552 were granted in 1985.
Clavulanic acid Specification
The Clavulanic acid with CAS registry number of 58001-44-8 is also known as 4-Oxa-1-azabicyclo[3.2.0]heptane-2-carboxylicacid, 3-(2-hydroxyethylidene)-7-oxo-, (2R,3Z,5R)-. The IUPAC name is (2R,3Z,5R)-3-(2-Hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. It belongs to product categories of Antibiotics. Its EINECS registry number is 261-069-2. In addition, the formula is C8H9NO5 and the molecular weight is 199.16. This chemical is a colorless needle-like crystal that soluble in water. It is a beta-lactamase inhibitor and used to overcome resistance in bacteria. What's more, it can be prepared by alkylation and chlorination decarboxylation reaction of 3-methylthiopropyllactam.
Physical properties about Clavulanic acid are: (1)ACD/BCF (pH 5.5): 1; (2)ACD/BCF (pH 7.4): 1; (3)ACD/KOC (pH 5.5): 1; (4)ACD/KOC (pH 7.4): 1; (5)#H bond acceptors: 6; (6)#H bond donors: 2; (7)#Freely Rotating Bonds: 3; (8)Index of Refraction: 1.644; (9)Molar Refractivity: 43.56 cm3; (10)Molar Volume: 120.2 cm3; (11)Surface Tension: 82.2 dyne/cm; (12)Density: 1.65 g/cm3; (13)Flash Point: 283.9 °C; (14)Enthalpy of Vaporization: 94.82 kJ/mol; (15)Boiling Point: 545.8 °C at 760 mmHg; (16)Vapour Pressure: 3.45E-14 mmHg at 25 °C.
Uses of Clavulanic acid: it is used to produce 2-(2-pyridyl)ethyl clavulanate by reaction with 2-pyridin-2-yl-ethanol. The reaction occurs with reagent dicyclohexylcarbodi-imide and solvent acetonitrile with ambient temperature. The yield is about 50%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing, gloves and eye/face protection. Avoid contact with skin and eyes. After using it, take off immediately all contaminated clothing. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1C2N(C1=O)C(C(=CCO)O2)C(=O)O
2. Isomeric SMILES: C1[C@@H]2N(C1=O)[C@H](/C(=C/CO)/O2)C(=O)O
3. InChI: InChI=1S/C8H9NO5/c10-2-1-4-7(8(12)13)9-5(11)3-6(9)14-4/h1,6-7,10H,2-3H2,(H,12,13)/b4-1-/t6-,7-/m1/s1
4. InChIKey: HZZVJAQRINQKSD-PBFISZAISA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1531mg/kg (1531mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 590, 1985. | |
| mouse | LD50 | intravenous | 4gm/kg (4000mg/kg) | "CRC Handbook of Antibiotic Compounds," Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980Vol. 4(1), Pg. 129, 1980. | |
| mouse | LD50 | oral | 4526mg/kg (4526mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 590, 1985. | |
| mouse | LD50 | subcutaneous | 2185mg/kg (2185mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 590, 1985. | |
| rat | LD50 | intraperitoneal | 1399mg/kg (1399mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 590, 1985. | |
| rat | LD50 | oral | 7936mg/kg (7936mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 590, 1985. | |
| rat | LD50 | subcutaneous | 1393mg/kg (1393mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 590, 1985. |
Related Products
- Clavulanic acid
- Clavulanic acid & Ticarcillin
- Clavulanic acid sodium salt
- 58002-62-3
- 5800-34-0
- 58-00-4
- 58004-19-6
- 58010-91-6
- 58011-68-0
- 58012-34-3
- 580-13-2
- 580-15-4
- 580-16-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View