-
Name
Cyclopropanecarbonitrile
- EINECS 226-836-8
- CAS No. 5500-21-0
- Article Data43
- CAS DataBase
- Density 0.96 g/cm3
- Solubility Miscible with water.
- Melting Point -25oC
- Formula C4H5N
- Boiling Point 135 °C at 760 mmHg
- Molecular Weight 67.0904
- Flash Point 32.8 °C
- Transport Information UN 3275 6.1/PG 2
- Appearance clear colorless to faintly yellow liquid
- Safety 23-26-36/37-45-36/37/39-16-7/9
- Risk Codes 10-23/24/25-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T,
T,  F
F
- Synonyms Cyanocyclopropane;Cyclopropylnitrile;Cyclopropyl cyanide;Cyclopropanenitrile;
- PSA 23.79000
- LogP 0.91998
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; sodium chloride In water; dimethyl sulfoxide | 98% |
| With sodium hydride In ethylene glycol; mineral oil at 20 - 80℃; for 0.5h; Solvent; Reagent/catalyst; | 97.2% |
| With potassium tert-butylate In tetrahydrofuran at -30℃; for 0.333333h; | 89% |

| Conditions | Yield |
|---|---|
| With sodium In ethylene glycol at 20 - 50℃; for 0.25h; Temperature; Solvent; Reagent/catalyst; | 98% |
| With ammonia; sodium amide; ferric nitrate | |
| With tetra(n-butyl)ammonium hydroxide In xylene at 40℃; |

| Conditions | Yield |
|---|---|
| With sodium hydride In ethylene glycol; mineral oil at 20 - 40℃; for 0.166667h; Solvent; Reagent/catalyst; | 96.5% |
-
-
58644-53-4
cyclopropyl isocyanide

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| at 520 - 550℃; under 0.01 Torr; | 92% |
| With 1,1-Diphenylethylene In various solvent(s) at 210℃; Rate constant; Thermodynamic data; ΔG(excit.) <250 deg C>; ΔH(excit.), ΔS(excit.); | 98 % Chromat. |

| Conditions | Yield |
|---|---|
| With sodium iodide; nickel dibromide; zinc In tetrahydrofuran at 0℃; for 42h; | 92% |

| Conditions | Yield |
|---|---|
| With thionyl chloride In toluene for 8h; Reflux; | 91% |
| With trichloromethyl chloroformate In various solvent(s) 0-5 deg C then heated to 60 deg C, 5 min; | 86% |
| With phosphorus pentoxide at 235℃; for 1h; Temperature; |
-
-
77100-86-8
2,4-dichlorobutanenitrile

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With tetraethylammonium tosylate In dimethyl sulfoxide for 10h; electrolysis; | 83% |
-
-
5332-06-9
4-bromobutanenitrile

-
A
-
628-22-8
4-hydroxy-1-butanitrile

-
B
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With oxygen; tetraethylammonium perchlorate In N,N-dimethyl-formamide at 20℃; electroreduction at -1.1 V; | A 10% B 67% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at -30℃; for 0.333333h; Title compound not separated from byproducts; | A 21% B n/a C n/a |
| With potassium tert-butylate In tetrahydrofuran at -30℃; Product distribution; Further Variations:; Reagents; Temperatures; | A 11% B n/a C n/a |

-
-
628-20-6
4-Chlorobutyronitrile

-
-
104-88-1
4-chlorobenzaldehyde

-
A
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at -30℃; for 0.333333h; Title compound not separated from byproducts; | A 16% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at -30℃; for 0.333333h; Title compound not separated from byproducts; | A 15% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at -30℃; | A n/a B n/a C 15% |

-
-
628-20-6
4-Chlorobutyronitrile

-
-
104-87-0
4-methyl-benzaldehyde

-
A
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at -30℃; for 0.333333h; Title compound not separated from byproducts; | A 14% B n/a C n/a |
| With potassium tert-butylate In tetrahydrofuran at -30℃; | A 14% B n/a C n/a |

-
-
628-20-6
4-Chlorobutyronitrile

-
-
141-52-6
sodium ethanolate

-
A
-
33563-82-5
4-ethoxy-butyronitrile

-
B
-
6228-73-5
Cyclopropancarbamid

-
C
-
5500-21-0
cyclopropropanecarbonitrile

-
-
5332-06-9
4-bromobutanenitrile

-
A
-
23662-46-6
cyclopropanecarboxamidine; cyclopropanecarboxamidine hydrobromide

-
B
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With ammonia; sodium amide; ferric nitrate |
-
-
25354-42-1
cyclopropyl trifluoromethanesulfonate

-
-
66997-38-4
tributylhexadecylphosphonium cyanide

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| In Cyclopentane 1) 22 h, 50 deg C, 2) 14 h, 70 deg C; | 98.8 % Turnov. |
-
-
93554-80-4
2-aminobutanenitrile hydrochloride

-
A
-
4476-02-2
2-hydroxybutanenitrile

-
B
-
4368-06-3
3-hydroxybutanenitrile

-
C
-
1190-76-7
(Z)-2-butenenitrile

-
D
-
4786-20-3
crotononitrile

-
E
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With perchloric acid; sodium nitrite for 24h; Product distribution; Mechanism; Ambient temperature; pH 3.5 or 1.4, also in HOAc/NaOAc, also (R)-2-aminobutanenitrile*HCl as substrate; | A 48.6 % Chromat. B 4.7 % Chromat. C 14.2 % Chromat. D 12.7 % Chromat. E 4.2 % Chromat. F 5.4 % Chromat. |


| Conditions | Yield |
|---|---|
| at 24.9℃; Thermodynamic data; ΔG0; |


-
-
67-64-1
acetone

-
A
-
17104-38-0, 43022-03-3
1-hydroxy-1-methyl-ethylium

-
B
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| at 24.9℃; Thermodynamic data; ΔG0; |



| Conditions | Yield |
|---|---|
| at 85℃; |
-
-
58644-53-4
cyclopropyl isocyanide

-
A
-
4786-20-3
but-2-enenitrile

-
B
-
109-75-1
but-3-enenitrile

-
C
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| at 999.84℃; under 0.0750075 Torr; Gas phase; | A 56 %Spectr. B 16 %Spectr. C 12 %Spectr. |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen In dichloromethane at 20℃; under 760.051 Torr; for 6h; Sealed tube; | 72 %Spectr. |
-
-
64-17-5
ethanol

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
63190-44-3
cyclopropanecarboximidic Acid Ethyl Ester Hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 20℃; | 100% |
| With hydrogenchloride In 1,4-dioxane at 20℃; | 100% |
| With hydrogenchloride In 1,4-dioxane at 20℃; for 12h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| at -196 - -64℃; for 0.166667h; Sealed tube; | 100% |
-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With arsenic pentafluoride at -196 - -64℃; for 0.166667h; Sealed tube; | 100% |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
209391-72-0
8-[Chloro-(toluene-4-sulfinyl)-methylene]-1,4-dioxa-spiro[4.5]decane

-
-
1381757-95-4
C20H24ClNO3S

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide | 99% |
-
-
178462-92-5
1-(2-(methylthio)-4-(trifluoromethyl)phenyl)ethanone

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In 2-methyltetrahydrofuran at 40℃; for 3h; Temperature; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: cyclopropropanecarbonitrile With cobalt pivalate; 1,1,3,3-Tetramethyldisiloxane; tert-butylisonitrile at 80℃; for 24h; Stage #2: With hydrogenchloride In diethyl ether at 20℃; for 0.5h; | 98% |
| With hydrogenchloride; dimethylsulfide borane complex 1.) reflux, 0.25 h, 2.) MeOH, reflux, 4h; Yield given. Multistep reaction; |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
98-80-6
phenylboronic acid

-
-
3481-02-5
cyclopropyl phenyl ketone

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; palladium diacetate; 2-(3,5-dimethyl-1H-pyrazol-1-yl)pyridine In water at 60℃; for 6h; | 98% |
| With [2,2]bipyridinyl; palladium diacetate; trifluoroacetic acid In tetrahydrofuran; water at 80℃; for 36h; Schlenk technique; Inert atmosphere; | 83% |
| With 1,2-bis(diphenylphosphino)ethane nickel(II) chloride; water; zinc(II) chloride In 1,4-dioxane at 80℃; for 8h; Inert atmosphere; | 82% |
-
-
67-56-1
methanol

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
77570-14-0
cyclopropanecarboximidic acid methyl ester monohydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether at 0℃; for 24h; | 97% |
| With hydrogenchloride at -10℃; for 5h; | 97% |
| With hydrogenchloride In diethyl ether at 0 - 20℃; for 17h; | 95.5% |
| With hydrogenchloride In 1,4-dioxane at 0 - 5℃; for 3h; | 783 mg |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
20295-34-5
cyclopropanecarbothioamide

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfide; diethyl amine hydrochloride In 1,4-dioxane; water at 55℃; for 42h; | 97% |
| With tetraphosphorus decasulfide In ethanol at 0 - 60℃; |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
75-65-0
tert-butyl alcohol

-
-
15924-91-1
N-(tert-butyl)cyclopropanecarboxamide

| Conditions | Yield |
|---|---|
| With silica boron-sulfuric acid nanoparticles at 20℃; for 0.25h; Ritter reaction; Neat (no solvent); | 97% |
| With silica-bonded N-propylsulphamic acid In neat (no solvent) at 80℃; for 1.33333h; Ritter Amidation; Green chemistry; | 96% |
| With bismuth(lll) trifluoromethanesulfonate; water at 100℃; Ritter reaction; | 95% |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
1521-51-3
rac-3-bromocyclohexene

-
-
1352832-71-3
1-(cyclohex-2-enyl)cyclopropanecarbonitrile

| Conditions | Yield |
|---|---|
| With n-butyllithium; ammonium chloride; diisopropylamine In tetrahydrofuran | 97% |

| Conditions | Yield |
|---|---|
| With sodium triethylborohydride In neat (no solvent) at 80℃; for 24h; Inert atmosphere; Glovebox; Green chemistry; | 97% |
-
-
63104-44-9
dimethyl 2,2-di(prop-2-ynyl)malonate

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
1516760-82-9
C15H17NO4

| Conditions | Yield |
|---|---|
| With η6-(naphtalene)(η5-cyclopentadienyl)-iron(II) hexafluorophosphate; tris(2,4,6-trimethoxyphenyl)phosphine In toluene at 120℃; Inert atmosphere; Schlenk technique; Microwave irradiation; | 96% |
-
-
70629-60-6, 73941-11-4, 16726-41-3
isopropylhydrazine hydrochloride

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In 1,4-dioxane at 120℃; for 15h; Inert atmosphere; Sealed tube; | 96% |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
870-63-3
prenyl bromide

-
-
95647-30-6
1-(3-methylbut-2-enyl)cyclopropanecarbonitrile

| Conditions | Yield |
|---|---|
| With n-butyllithium; ammonium chloride; diisopropylamine In tetrahydrofuran | 95% |
-
-
98-85-1, 13323-81-4
1-Phenylethanol

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
78172-92-6
N-(1-phenylethyl)cyclopropanecarboxamide

| Conditions | Yield |
|---|---|
| With silica boron-sulfuric acid nanoparticles at 20℃; for 0.166667h; Ritter reaction; Neat (no solvent); | 95% |
-
-
63243-76-5
2-amino-5-bromobenzoic acid,ethyl ester

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane Reflux; | 95% |
| With hydrogenchloride In 1,4-dioxane; water Reflux; |
-
-
541-16-2
t-butyl malonate

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
15924-91-1
N-(tert-butyl)cyclopropanecarboxamide

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80℃; for 2h; Ritter Amidation; | 95% |
-
-
79762-54-2
6-bromo-1H-indazole

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With N-Xantphos Pd G4; lithium hexamethyldisilazane In tetrahydrofuran at 20℃; for 0.25h; Inert atmosphere; Sealed tube; | 95% |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
57297-29-7
cyclopropylcarboxamidine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: cyclopropropanecarbonitrile With hydrogenchloride; methanol In toluene at 15 - 23℃; for 18h; Stage #2: With ammonia In methanol; toluene at 5 - 25℃; for 1.16667h; | 94% |
| Stage #1: cyclopropropanecarbonitrile With hydrogenchloride In ethanol at 20℃; for 24h; Stage #2: With ammonia In ethanol at 23℃; Cooling with ice; | 79.5% |
| Stage #1: cyclopropropanecarbonitrile With hydrogenchloride; methanol In diethyl ether at 0℃; for 2h; Stage #2: With ammonia In methanol for 1h; Cooling with ice; | 67% |
-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
51285-13-3
cyclopropanecarboxamide oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine In ethanol; water at 80℃; | 94% |
| With hydroxylamine hydrochloride; sodium hydrogencarbonate In isopropyl alcohol at 80 - 85℃; | 70% |
| With hydroxylamine hydrochloride; sodium hydrogencarbonate In ethanol for 6h; Reflux; | 52% |
-
-
613-92-3
N-hydroxybenzenecarboximidamide

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
884637-65-4
5-cyclopropyl-3-phenyl-1,2,4-oxadiazole

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; zinc(II) chloride In N,N-dimethyl-formamide at 80℃; Inert atmosphere; | 94% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
1351078-23-3
C12H27NO6P2

| Conditions | Yield |
|---|---|
| With bis(cyclopentadienyl)titanium dichloride; methyloxirane; zinc In tetrahydrofuran for 20h; Inert atmosphere; Reflux; | 94% |
-
-
91-01-0
1,1-Diphenylmethanol

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
545437-24-9
N-(diphenylmethyl)cyclopropylcarboxamide

| Conditions | Yield |
|---|---|
| With silica-bonded N-propylsulphamic acid In neat (no solvent) at 80℃; for 0.416667h; Ritter Amidation; Green chemistry; | 94% |
-
-
292638-84-7
styrene

-
-
5500-21-0
cyclopropropanecarbonitrile

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; bis(4-methoxyphenyl)selenide In hexane; water at 20℃; for 24h; Sealed tube; | 94% |

-
-
101-81-5
Diphenylmethane

-
-
5500-21-0
cyclopropropanecarbonitrile

-
-
545437-24-9
N-(diphenylmethyl)cyclopropylcarboxamide

| Conditions | Yield |
|---|---|
| With manganese(II) acetate dihydrate; trifluoroacetic acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,2-dichloro-ethane at 90℃; for 12h; Inert atmosphere; | 94% |
| With manganese(III) triacetate dihydrate; trifluoroacetic acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,2-dichloro-ethane at 90℃; for 12h; Schlenk technique; Inert atmosphere; | 90% |
Cyclopropanecarbonitrile Specification
The Cyclopropanecarbonitrile, with the CAS registry number 5500-21-0 and EINECS registry number 226-836-8, is a kind of clear colorless to faintly yellow liquid. And the molecular formula of this chemical is C4H5N. It belongs to the following product categories: Pharmaceutical Intermediates; Cyclopropanes; Simple 3-Membered Ring Compounds.
The physical properties of Cyclopropanecarbonitrile are as following: (1)ACD/LogP: -0.08; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.08; (4)ACD/LogD (pH 7.4): -0.08; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 21.54; (8)ACD/KOC (pH 7.4): 21.54; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 23.79 Å2; (13)Index of Refraction: 1.442; (14)Molar Refractivity: 18.43 cm3; (15)Molar Volume: 69.6 cm3; (16)Polarizability: 7.3×10-24cm3; (17)Surface Tension: 33.8 dyne/cm; (18)Density: 0.96 g/cm3; (19)Flash Point: 32.8 °C; (20)Enthalpy of Vaporization: 35.55 kJ/mol; (21)Boiling Point: 135 °C at 760 mmHg; (22)Vapour Pressure: 7.88 mmHg at 25°C.
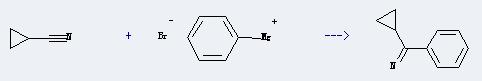
Uses of Cyclopropanecarbonitrile: It can react with phenylmagnesium bromide to produce cyclopropyl-phenyl ketone-imine. This reaction will need solvent diethyl ether. The reaction time is 2 hours with ambient temperature, and the yield is about 65%.
You should be cautious while dealing with this chemical. It is a flammable chemcial which is toxic by inhalation, in contact with skin and if swallowed. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; Keep away from sources of ignition - No smoking; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: N#CC1CC1
(2)InChI: InChI=1/C4H5N/c5-3-4-1-2-4/h4H,1-2H2
(3)InChIKey: AUQDITHEDVOTCU-UHFFFAOYAL
Related Products
- Cyclopropanecarbonitrile
- Cyclopropanecarbonitrile,1-(3-chlorophenyl)-
- 55004-77-8
- 55011-44-4
- 550-21-0
- 55021-17-5
- 550-24-3
- 55025-15-5
- 55025-80-4
- 55025-91-7
- 5502-74-9
- 55028-72-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View