-
Name
Cytisine
- EINECS 207-616-0
- CAS No. 485-35-8
- Article Data9
- CAS DataBase
- Density 1.24 g/cm3
- Solubility Soluble to 100 mM in Water
- Melting Point 154-156 °C(lit.)
- Formula C11H14N2O
- Boiling Point 413 °C at 760 mmHg
- Molecular Weight 190.245
- Flash Point 203.6 °C
- Transport Information
- Appearance Off-white to tan crystalline solid
- Safety 26-28-36/37-45
- Risk Codes 25-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 1,5-Methano-8H-pyrido[1,2-a][1,5]diazocin-8-one,1,2,3,4,5,6-hexahydro-, (1R)-;Cytisine (6CI,8CI);(-)-Cytisine;Baptitoxin;Baptitoxine;Cytisin;Cytiton;Cytitone;Laburnin;Sophorin;Sophorine;Tabex;Tsitafat;Ulexin;Ulexine;1,5-Methano-8H-pyrido[1,2-a][1,5]diazocin-8-one,1,2,3,4,5,6-hexahydro-, (1R,5S)-;
- PSA 34.03000
- LogP 0.88380
Synthetic route
-
-
667940-15-0
(6R,7R,9R)-(-)-N-benzyloxycarbonyl-cytisine

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran for 3h; Heating; | 78% |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| With potassium carbonate; thiophenol In N,N-dimethyl-formamide; acetonitrile at 45℃; for 0.5h; | 96% |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Stage #1: (1R,9R)-11-tert-butoxycarbonyl-7,11-diazatricyclo[7.3.1.02,7]tridec-2,4-dien-6,10-dione With methanol; sodium tetrahydroborate at 0 - 20℃; for 2h; Stage #2: With triethylsilane; boron trifluoride diethyl etherate In dichloromethane at -78 - 20℃; for 15h; | 79% |
-
-
206554-22-5
(3S,5R)-(+)-3-acetoxymethyl-5-formyl-1-piperidine-1-carbvoxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: (-)-B-methoxydiisopinocampheylborane / diethyl ether / 1.25 h / -78 - 20 °C 1.2: diethyl ether / -78 - 20 °C / Acid hydrolysis 1.3: 76 percent / aq. NaOH; aq. H2O2 / 3 h / Heating 2.1: 99 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 3.1: 87 percent / NaN3 / dimethylformamide / 2 h / 80 °C 4.1: Ph3P / tetrahydrofuran 4.2: 66 percent / H2O 5.1: 89 percent / Et3N; DMAP / CH2Cl2 / 4.5 h / 0 - 20 °C 6.1: 79 percent / Cl2Ru(CHPh)(PCy3)2 / CH2Cl2 / 12 h / Heating 7.1: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 8.1: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 9.1: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 10.1: 50 percent / DDQ / dioxane / 4 h / 110 °C 11.1: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
206761-38-8
(3S,5R)-3-acetoxymethyl-5-hydroxymethylpiperidine-1-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: (-)-B-methoxydiisopinocampheylborane / diethyl ether / 1.25 h / -78 - 20 °C 1.2: diethyl ether / -78 - 20 °C / Acid hydrolysis 1.3: 76 percent / aq. NaOH; aq. H2O2 / 3 h / Heating 2.1: 99 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 3.1: 87 percent / NaN3 / dimethylformamide / 2 h / 80 °C 4.1: Ph3P / tetrahydrofuran 4.2: 66 percent / H2O 5.1: 89 percent / Et3N; DMAP / CH2Cl2 / 4.5 h / 0 - 20 °C 6.1: 79 percent / Cl2Ru(CHPh)(PCy3)2 / CH2Cl2 / 12 h / Heating 7.1: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 8.1: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 9.1: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 10.1: 50 percent / DDQ / dioxane / 4 h / 110 °C 11.1: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-16-1
(3S,5R,1'S)-(-)-3-acetoxymethyl-5-(1'-hydroxybut-3'-enyl)-1-piperidine-1-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: 99 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 2.1: 87 percent / NaN3 / dimethylformamide / 2 h / 80 °C 3.1: Ph3P / tetrahydrofuran 3.2: 66 percent / H2O 4.1: 89 percent / Et3N; DMAP / CH2Cl2 / 4.5 h / 0 - 20 °C 5.1: 79 percent / Cl2Ru(CHPh)(PCy3)2 / CH2Cl2 / 12 h / Heating 6.1: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 7.1: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 8.1: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 9.1: 50 percent / DDQ / dioxane / 4 h / 110 °C 10.1: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-11-6
(3S,5R,1'R)-(-)-3-acetoxymethyl-5-(1'-azidobut-3'-enyl)-1-piperidine-1-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: Ph3P / tetrahydrofuran 1.2: 66 percent / H2O 2.1: 89 percent / Et3N; DMAP / CH2Cl2 / 4.5 h / 0 - 20 °C 3.1: 79 percent / Cl2Ru(CHPh)(PCy3)2 / CH2Cl2 / 12 h / Heating 4.1: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 5.1: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 6.1: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 7.1: 50 percent / DDQ / dioxane / 4 h / 110 °C 8.1: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-18-3
(3S,5R)-3-Acetoxymethyl-5-((R)-1-amino-but-3-enyl)-piperidine-1-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 89 percent / Et3N; DMAP / CH2Cl2 / 4.5 h / 0 - 20 °C 2: 79 percent / Cl2Ru(CHPh)(PCy3)2 / CH2Cl2 / 12 h / Heating 3: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 4: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 5: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 6: 50 percent / DDQ / dioxane / 4 h / 110 °C 7: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-10-5
(3S,5R,1'S)-(+)-3-acetoxymethyl-5-(1'-methanesulfonyloxybut-3'-enyl)-1-piperidine-1-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: 87 percent / NaN3 / dimethylformamide / 2 h / 80 °C 2.1: Ph3P / tetrahydrofuran 2.2: 66 percent / H2O 3.1: 89 percent / Et3N; DMAP / CH2Cl2 / 4.5 h / 0 - 20 °C 4.1: 79 percent / Cl2Ru(CHPh)(PCy3)2 / CH2Cl2 / 12 h / Heating 5.1: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 6.1: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 7.1: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 8.1: 50 percent / DDQ / dioxane / 4 h / 110 °C 9.1: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-08-1
(3S,5R,1'R)-(+)-3-acetoxymethyl-5-(1'-acryloylaminobut-3'-enyl)-1-piperidine-1-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 79 percent / Cl2Ru(CHPh)(PCy3)2 / CH2Cl2 / 12 h / Heating 2: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 3: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 4: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 5: 50 percent / DDQ / dioxane / 4 h / 110 °C 6: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
485-35-8, 15191-27-2, 55821-72-2
1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1,2-a][1,5]diazocin-8-one

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid |
-
-
667940-14-9
(6R,7R,9R)-(+)-N-benzyloxycarbonyl-5,6-dihydro-cytisine

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 50 percent / DDQ / dioxane / 4 h / 110 °C 2: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-12-7
(2R,3'R,5'S)-(+)-5-hydroxymethyl-6-oxo-1,2,3,6,3',4',5',6'-octahydro-2'H-[2,3']-bipyridinyl-1'-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 2: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 3: 50 percent / DDQ / dioxane / 4 h / 110 °C 4: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-07-0
(2R,3'R,5'S)-(+)-5-acetoxymethyl-6-oxo-1,2,3,6,3',4',5',6'-octahydro-2'H-[2,3']-bipyridinyl-1'-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 98 percent / NaOH / tetrahydrofuran / 2 h / 20 °C 2: 67 percent / Et3N; DMAP / CH2Cl2 / 1.5 h / 20 °C 3: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 4: 50 percent / DDQ / dioxane / 4 h / 110 °C 5: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |
-
-
667940-13-8
(2R,3'R,5'S)-(+)-5-methanesulfonyloxymethyl-6-oxo-1,2,3,6,3',4',5',6'-octahydro-2'H-[2,3']-bipyridinyl-1'-carboxylic acid benzyl ester

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 89 percent / NaH / tetrahydrofuran / 0 - 20 °C 2: 50 percent / DDQ / dioxane / 4 h / 110 °C 3: 78 percent / HCl / tetrahydrofuran / 3 h / Heating View Scheme |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine; dmap / dichloromethane / 20 h 2.1: triethylamine / tetrahydrofuran / 5 h / 20 °C 3.1: methanol; sodium tetrahydroborate / 2 h / 0 - 20 °C 3.2: 15 h / -78 - 20 °C View Scheme |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / tetrahydrofuran / 5 h / 20 °C 2.1: methanol; sodium tetrahydroborate / 2 h / 0 - 20 °C 2.2: 15 h / -78 - 20 °C View Scheme |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 °C 2.1: triethylamine / dichloromethane / 1 h / 0 °C 3.1: chloroform / 6.5 h / Reflux 3.2: 9 h / 23 °C 4.1: thiophenol; potassium carbonate / acetonitrile; N,N-dimethyl-formamide / 0.5 h / 45 °C View Scheme |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / dichloromethane / 1 h / 0 °C 2.1: chloroform / 6.5 h / Reflux 2.2: 9 h / 23 °C 3.1: thiophenol; potassium carbonate / acetonitrile; N,N-dimethyl-formamide / 0.5 h / 45 °C View Scheme |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: chloroform / 6.5 h / Reflux 1.2: 9 h / 23 °C 2.1: thiophenol; potassium carbonate / acetonitrile; N,N-dimethyl-formamide / 0.5 h / 45 °C View Scheme |
-
-
485-35-8
cytisine

-
-
19634-60-7
Nitrosocytisine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite at 20℃; for 288h; Nitrosation; | 100% |
| With potassiuim nitrosodisulfonate; hydroxylamine hydrochloride; sodium carbonate In pyridine for 0.25h; | 91% |
-
-
108-24-7
acetic anhydride

-
-
485-35-8
cytisine

-
-
6018-52-6
(-)-(1R,5S)-N-acetyl-1,2,3,4,5,6-hexahydro-1,5-methano-pyrido-[1,2-a][1,5]diazocin-8-one

| Conditions | Yield |
|---|---|
| With dmap In pyridine at 20℃; for 12h; Acetylation; | 100% |
| 88.4% |

| Conditions | Yield |
|---|---|
| In benzene at 80℃; | 100% |
-
-
4411-26-1
1-adamantylisothiocyanate

-
-
485-35-8
cytisine

-
-
1621257-20-2
N-1-adamantylcytisine-12-thiocarbamide

| Conditions | Yield |
|---|---|
| In benzene at 80℃; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
485-35-8
cytisine

-
-
207390-62-3
8-oxo-1,5,6,8-tetrahydro-2H,4H-1,5-methano-pyrido[1,2-a][1,5]diazocine-3-carboxylic acid tert-butyl ester [N-tboccytisine]

| Conditions | Yield |
|---|---|
| With sodium carbonate In tetrahydrofuran; water at 20℃; for 48h; | 99% |
| With sodium carbonate In tetrahydrofuran at 20℃; for 24h; | 96% |
| With sodium carbonate In tetrahydrofuran; water for 72h; | 93% |
-
-
100-39-0
benzyl bromide

-
-
485-35-8
cytisine

-
-
78867-61-5, 109667-42-7
(1R,9S)-11-benzyl-7,11-diazatricyclo[7.3.1.0]trideca-2,4-dien-6-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 20h; Reflux; | 99% |
| With potassium carbonate In acetonitrile for 5h; Heating; | 94% |
| With potassium carbonate In acetonitrile for 5h; Reflux; Inert atmosphere; | 94% |
| With sodium carbonate In dichloromethane; water for 4h; Heating; | 77% |

| Conditions | Yield |
|---|---|
| In pentan-1-ol at 137℃; | 98% |

| Conditions | Yield |
|---|---|
| In benzene at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| In benzene at 20℃; | 98% |
-
-
101-68-8
di(4-isocyanatophenyl)methane

-
-
485-35-8
cytisine

-
-
1620659-65-5
N,N'-[methylene-bis(4,1-phenylene)]dicytisinecarboxamide

| Conditions | Yield |
|---|---|
| In benzene at 80℃; | 98% |
-
-
85234-59-9
2-(4-phenoxybutyl)oxirane

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| In methanol at 90℃; for 8h; | 98% |

| Conditions | Yield |
|---|---|
| With trimethylsilylazide In methanol at 20℃; for 24h; Ugi Condensation; | 98% |


| Conditions | Yield |
|---|---|
| With trimethylsilylazide In methanol at 20℃; for 24h; Ugi Condensation; | 98% |

-
-
38870-89-2
Methoxyacetyl chloride

-
-
485-35-8
cytisine

-
-
881853-39-0
(1R,5S)-N-(2-methoxyacetyl)-1,2,3,4,5,6-hexahydro-1,5-methanopyrido[1,2-a]diazocin-8-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 18h; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Acylation; Heating; | 96% |
-
-
79-03-8
propionyl chloride

-
-
485-35-8
cytisine

-
-
474116-07-9
(1R,5S)-3-propionyl-3,4,5,6-tetrahydro-1H-1,5-methanopyrido[1,2-a][1,5]diazocin-8(2H)-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 96% |
| With triethylamine In dichloromethane at 0 - 22℃; for 3h; | 12.3 g |

| Conditions | Yield |
|---|---|
| In benzene at 35 - 40℃; | 96% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 72h; | 96% |

-
-
485-35-8
cytisine

| Conditions | Yield |
|---|---|
| In ethanol at 90℃; for 20h; | 96% |
| In ethanol at 90℃; for 20h; | 96% |
-
-
485-35-8
cytisine

-
-
109-94-4
formic acid ethyl ester

-
-
53007-06-0
(1R,5S)-8-oxo-1,5,6,8-tetrahydro-2H-1,5-methanopyrido[1,2-a][1,5]diazocine-3(4H)-carbaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine for 19h; Reflux; | 96% |

| Conditions | Yield |
|---|---|
| With trimethylsilylazide In methanol at 20℃; for 24h; Ugi Condensation; | 96% |

Cytisine Chemical Properties
Following is the structure of Cytisine (CAS NO.485-35-8):
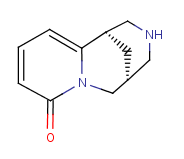
Molecular Formula: C11H14N2O
Molecular Weight: 190.27
EINECS: 207-616-0
Molar Refractivity: 54.04 cm3
Molar Volume: 153.2 cm3
Density: 1.24 g/cm3
Flash Point: 203.6 °C
Index of Refraction: 1.623
Melting Point: 154-156 °C
Surface Tension: 50.8 dyne/cm
Enthalpy of Vaporization: 66.58 kJ/mol
Boiling Point: 413 °C at 760 mmHg
Vapour Pressure: 4.95E-07 mmHg at 25 °C
Appearance of Cytisine (CAS NO.485-35-8): Off-White to Tan Crystalline Solid
Product Categories of Cytisine (CAS NO.485-35-8): Heterocyclic Compounds; Neurochemicals; Nicotine Derivatives
Canonical SMILES: C1C2CNCC1C3=CC=CC(=O)N3C2
InChI: InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2
InChIKey: ANJTVLIZGCUXLD-UHFFFAOYSA-N
Cytisine Uses
Toxic priniciple in seed of Laburnum anagyroides and other Leguminosae. A neuronal nicotinic acetylcholine agonist.
Cytisine Toxicity Data With Reference
| 1. | scu-rat LDLo:20 mg/kg | 85IXA4 Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif Bovet, D., andF. Bovet-Nitti,New York, NY.: S. Karger,1948,589. | ||
| 2. | orl-mus LD50:101 mg/kg | BJPCBM British Journal of Pharmacology. 35 (1969),161. | ||
| 3. | ipr-mus LD50:9400 µg/kg | BJPCBM British Journal of Pharmacology. 35 (1969),161. | ||
| 4. | ivn-mus LD50:1730 µg/kg | BJPCBM British Journal of Pharmacology. 35 (1969),161. | ||
| 5. | inv-cat LD50:400 µg/kg | ITOBAO Izvestiya Akademii Nauk Tadzhikskoi SSR, Otdelenie Biologicheskikh Nauk.(2),(1978),104. |
Cytisine Consensus Reports
Reported in EPA TSCA Inventory.
Cytisine Safety Profile
Poison by ingestion, intravenous and intraperitoneal routes. A toxin found in some plants. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes:  T
T
Risk Statements: 25-36/37/38
R25 :Toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-28-36/37-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S28:After contact with skin, wash immediately with plenty of soap-suds.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 2811 6.1/PG 3
WGK Germany: 3
RTECS: HA4025000
HazardClass: 6.1(b)
PackingGroup: III
Cytisine Specification
Cytisine , its cas register number 485-35-8. It also can be called (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-methanopyrido[1,2-a][1,5]diazocin-8-one ; and 1,5-methano-8H-pyrido[1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-, (1R,5S)- .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View