-
Name
DL-3-Aminoisobutyric acid
- EINECS 234-154-7
- CAS No. 10569-72-9
- Article Data50
- CAS DataBase
- Density 1.105 g/cm3
- Solubility
- Melting Point 179-182 °C(lit.)
- Formula C4H9NO2
- Boiling Point 223.6 °C at 760 mmHg
- Molecular Weight 103.121
- Flash Point 89 °C
- Transport Information
- Appearance white crystal or crystalline powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (1)-3-Amino-2-methylpropionic acid;(±)-b-Aminoisobutyric acid;DL-Beta-Aminoisobutyric Acid;H-Aib-OH;
- PSA 63.32000
- LogP 0.36610
Synthetic route
-
-
696-04-8
5,6-dihydrothymine

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With phosphoric acid; dihydropyrimidinase; N-carbamoyl- -alanine amidohydrolase; nickel dichloride In aq. phosphate buffer at 30℃; pH=8; Enzymatic reaction; |
-
-
95503-63-2
2-benzyl-4-methylisoxazolidin-5-one

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In 1,4-dioxane; water at 85℃; for 16h; | 95% |
-
-
95503-68-7, 95503-69-8
4-methyl-N-(S)-1-phenylethylisoxazolidin-5-one

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In 1,4-dioxane; water at 85℃; for 16h; | 87% |
-
-
131932-58-6
trans-1-benzoyl-2-tert-butyl-3,5-dimethylperhydropyrimidin-4-one

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 180℃; for 8h; | 69% |
-
-
66839-25-6
2-methyl-3-benzylaminopropanoic acid

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol | |
| With hydrogen; PdCl2/C In acetic acid |

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In tetrahydrofuran; methanol |

-
B
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With naphthalene; sodium In 1,2-dimethoxyethane at -78℃; for 0.5h; | A 31 mg B n/a |

-
B
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With naphthalene; sodium In 1,2-dimethoxyethane at -78℃; for 0.5h; | A 67 mg B n/a |
-
-
1572-99-2
ethyl 2-cyanopropionate

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid; platinum Hydrogenation.wiederholtes Eindampfen des Reaktionsprodukts mit Wasser; |
-
-
55125-15-0
N,N,O-Tris-(trimethylsilyl)-β-aminoisobuttersaeure

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With water; triethylamine In tetrahydrofuran; pentane at 20℃; |
-
-
97-63-2
methyl methacrylate

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With ethanol; ammonia; water; hydroquinone at 125 - 130℃; und Erwaermen des Reaktionsprodukts mit wss.HCl; |
-
-
14678-48-9
β-amino-α-methyl propionic acid methyl ester

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With lithium hydroxide In water |

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
1948-56-7
α-aminoacrylic acid

-
C
-
32909-71-0
2-amino-4-hydroxypentanedioic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 110℃; for 17h; Hydrolysis; |

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With Canyon Diablo iron meteorites at 140℃; for 24h; Reagent/catalyst; Temperature; |
-
-
126-98-7
methacrylonitrile

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; sulfur trioxide |
-
-
107-10-8
propylamine

-
-
64-18-6
formic acid

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
302-72-7
rac-Ala-OH

-
C
-
2835-81-6
2-aminobutanoic acid

-
D
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); Further byproducts given; | A 9.8% B 3.4% C 0.9% D 8.1% |

-
-
107-10-8
propylamine

-
-
64-18-6
formic acid

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
2835-81-6
2-aminobutanoic acid

-
C
-
107-95-9
3-amino propanoic acid

-
D
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); Further byproducts given; | A 9.8% B 0.9% C 3.4% D 8.1% |

-
-
84796-57-6
Sodium; 2-methyl-3-ureido-propionate

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With hydrogen cation Yield given; |

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With hydrogen iodide; acetic acid |

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With water wiederholtes Eindampfen; |
-
-
102076-62-0
(benzoylamino-methyl)-methyl-malonic acid diethyl ester

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide |
-
-
80-62-6
methacrylic acid methyl ester

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| (i) NH3, MeOH, (ii) aq. HCl; Multistep reaction; |
-
-
64-18-6
formic acid

-
-
107-11-9
1-amino-2-propene

-
A
-
107-10-8
propylamine

-
B
-
10569-72-9
DL-3-aminoisobutyric acid

-
C
-
56-40-6
glycine

-
D
-
107-95-9
3-amino propanoic acid

-
E
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| With hydrogen; oxygen In water for 3h; Product distribution; various unsaturated amines; investigation of the direct carboxylation of C=C bond, the effect of formic acid concentration as well as the flame composition on product(s); radical mechanism is proposed; | A n/a B 38% C n/a D n/a E 5% |

-
-
632-07-5
2-cyanopropanoic acid

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| Hydrogenation; |
-
-
7664-93-9
sulfuric acid

-
-
1572-99-2
ethyl 2-cyanopropionate

-
-
64-19-7
acetic acid

-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| beim folgenden Verseifen.Hydrogenation; |

-
-
107-10-8
propylamine

-
-
64-18-6
formic acid

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
56-40-6
glycine

-
C
-
2835-81-6
2-aminobutanoic acid

-
D
-
107-95-9
3-amino propanoic acid

-
E
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; Product distribution; contact glow discharge electrolysis; variation of pH, effect of time; | A 9.8% B 0.2% C 0.9% D 3.4% E 8.1% |


| Conditions | Yield |
|---|---|
| With hydrogen azide for 5h; Quantum yield; Ambient temperature; Irradiation; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 100% |
| With thionyl chloride for 4.5h; | 85% |
| Stage #1: ethanol; DL-3-aminoisobutyric acid With thionyl chloride at 0 - 90℃; for 6h; Stage #2: With ammonium hydroxide In dichloromethane at 20℃; for 2h; |
-
-
67-56-1
methanol

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
14678-48-9
β-amino-α-methyl propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride at 20℃; | 100% |
| With thionyl chloride for 3h; Reflux; | |
| With thionyl chloride at 0 - 20℃; |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
66164-10-1
3-amino-2-methylpropan-1-ol hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid With borane-THF In tetrahydrofuran at 20℃; for 20.5h; Reflux; Stage #2: With hydrogenchloride In 1,4-dioxane; diethyl ether for 0.333333h; | 100% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
16948-10-0
N-tert-butoxycarbonyl-3-amino-2(RS)-methylpropionic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tert-butyl alcohol In water at 30℃; for 18h; | 99% |
| With sodium hydroxide In tetrahydrofuran at 20 - 30℃; for 18h; | 97% |
| Stage #1: DL-3-aminoisobutyric acid; di-tert-butyl dicarbonate With sodium hydroxide In 1,4-dioxane; water at 5℃; for 0.916667h; Stage #2: With hydrogenchloride In 1,4-dioxane; water | 96% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
606-21-3
1-chloro-2,6-dinitrobenzene

-
-
189939-44-4
3-(2,6-Dinitro-phenylamino)-2-methyl-propionic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol for 6h; Heating; | 98% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
2905-62-6
3,5-dichlorobenzoyl chloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 1h; | 93% |
-
-
67-56-1
methanol

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
88512-06-5
β-aminoisobutyric acid methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; for 6h; | 90% |
| With trimethylsilyl isocyanate at 0 - 20℃; for 48h; Inert atmosphere; | 75% |
| With thionyl chloride at -10℃; for 1h; Heating / reflux; | |
| With thionyl chloride at 0℃; for 18h; Inert atmosphere; Reflux; |

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; DL-3-aminoisobutyric acid With trifluoroacetic acid at 150℃; for 0.0833333h; Sealed tube; Inert atmosphere; Microwave irradiation; Stage #2: With sulfuric acid In methanol for 4h; Reflux; | 90% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
39637-74-6
(1S)-(-)-camphanic chloride

-
-
88099-59-6, 88099-60-9, 98974-77-7
(2RS)-3-camphanoylamino-2-methylpropanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene for 3h; | 89% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
58521-61-2
3-methyl-2-azetidinone

| Conditions | Yield |
|---|---|
| With sulfenamide (5); triphenylphosphine In acetonitrile at 80℃; for 2h; | 89% |
| With tris(2-oxo-3-oxazolinyl)phosphine oxide; triethylamine In acetonitrile for 6h; Heating; | 75% |
| With tris(1,3-dihydro-2-oxobenzoxazolin-3-yl) phosphine oxide; triethylamine In acetonitrile for 6h; Heating; | 71% |
| With 2-chloro-1-methyl-pyridinium iodide; triethylamine In acetonitrile for 2h; Heating; | 57% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
122-78-1
phenylacetaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid; phenylacetaldehyde In water at 20℃; Stage #2: tert-butylisonitrile In water at 20℃; Ugi reaction; | 88% |
| In methanol for 24h; Ambient temperature; |

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
88333-03-3, 10340-91-7
Benzyl isocyanide

-
-
123-38-6
propionaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid; propionaldehyde In water at 20℃; Stage #2: Benzyl isocyanide In water at 20℃; Ugi reaction; | 84% |

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
78-84-2
isobutyraldehyde

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid; isobutyraldehyde In water at 20℃; Stage #2: tert-butylisonitrile In water at 20℃; Ugi reaction; | 82% |

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
88333-03-3, 10340-91-7
Benzyl isocyanide

-
-
78-84-2
isobutyraldehyde

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid; isobutyraldehyde In water at 20℃; Stage #2: Benzyl isocyanide In water at 20℃; Ugi reaction; | 82% |

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
88333-03-3, 10340-91-7
Benzyl isocyanide

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid; isovaleraldehyde In water at 20℃; Stage #2: Benzyl isocyanide In water at 20℃; Ugi reaction; | 82% |

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid; isovaleraldehyde In water at 20℃; Stage #2: tert-butylisonitrile In water at 20℃; Ugi reaction; | 81% |

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
59741-04-7
2-methoxy-5-methylphenyl isocyanate

-
-
1270915-86-0
3-(3-(2-methoxy-5-methylphenyl)ureido)-2-methylpropanoic acid

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 81% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
383-63-1
ethyl trifluoroacetate,

-
-
101642-73-3
2-methyl-3-(2,2,2-trifluoroacetamido)propanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: DL-3-aminoisobutyric acid With triethylamine In methanol for 0.0833333h; Stage #2: ethyl trifluoroacetate, In methanol for 24h; | 81% |
-
-
67-56-1
methanol

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
141109-47-9
[di(propan-2-yl)amino](oxo)acetyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: methanol; DL-3-aminoisobutyric acid With thionyl chloride at 0 - 20℃; Stage #2: [di(propan-2-yl)amino](oxo)acetyl chloride With triethylamine In dichloromethane at 0 - 20℃; for 6h; | 78% |

-
-
40397-98-6
2,5-dimethylphenyl isocyanate

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
1337962-17-0
3-(3-(2,5-dimethylphenyl)ureido)-2-methylpropanoic acid

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 76% |
-
-
591-50-4
iodobenzene

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
73849-53-3
N-phenyl-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(I) bromide; 1,1'-bi-2-naphthol In N,N-dimethyl-formamide at 40℃; for 10h; | 72% |
-
-
85-44-9
phthalic anhydride

-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
24431-49-0
3-(1,3-dioxoisoindolin-2-yl)-2-methylpropanoic acid

| Conditions | Yield |
|---|---|
| With triethylamine In toluene Reflux; | 70% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate In water; N,N-dimethyl-formamide at 150℃; for 4h; Microwave irradiation; | 70% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
17341-93-4
2,2,2-Trichloroethyl chloroformate

-
-
1417602-90-4
2-methyl-3-((2,2,2-trichloroethoxy)carbonylamino)propanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0 - 20℃; for 2.5h; | 68% |
-
-
10569-72-9
DL-3-aminoisobutyric acid

-
-
40411-27-6
4-chloro-2-isocyanato-1-methylbenzene

-
-
1337962-18-1
3-(3-(5-chloro-2-methylphenyl)ureido)-2-methylpropanoic acid

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 61% |
DL-3-Aminoisobutyric acid Specification
The CAS registry number of DL-3-Aminoisobutyric acid is 10569-72-9. The IUPAC name is 3-amino-2-methylpropanoic acid. In addition, the molecular formula is C4H9NO2. What's more, it belongs to the class of Amino Acids. Besides, it should be stored in sealed container, and put in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: -0.51; (2)ACD/LogD (pH 5.5): -3.01; (3)ACD/LogD (pH 7.4): -3.01; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 3; (9)#H bond donors: 3; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 29.54 Å2; (12)Index of Refraction: 1.462; (13)Molar Refractivity: 25.63 cm3; (14)Molar Volume: 93.2 cm3; (15)Polarizability: 10.16 ×10-24cm3; (16)Surface Tension: 43.5 dyne/cm; (17)Density: 1.105 g/cm3; (18)Flash Point: 89 °C; (19)Enthalpy of Vaporization: 50.68 kJ/mol; (20)Boiling Point: 223.6 °C at 760 mmHg; (21)Vapour Pressure: 0.0352 mmHg at 25°C.
Preparation of DL-3-Aminoisobutyric acid: it can be prepared by 2-benzyl-4-methylisoxazolidin-5-one. This reaction will need reagents H2 and Pd/C, and solvents dioxane and H2O. The reaction time is 16 hours at reaction temperature of 85 °C. The yield is about 95%.
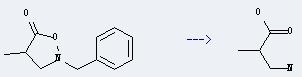
Uses of DL-3-Aminoisobutyric acid: it can be used to get 3-methyl-azetidin-2-one. This reaction will need reagents 2-chloro-1-methylpyridinium iodide and Et3N, and solvent acetonitrile. The reaction time is 2 hours by heating. The yield is about 57%.

When you are using this chemical, please be cautious about it as the following:
During using it, you should avoid contact with skin and eyes. In addition, do not breathe dust.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)C(C)CN
(2)InChI: InChI=1/C4H9NO2/c1-3(2-5)4(6)7/h3H,2,5H2,1H3,(H,6,7)
(3)InChIKey: QCHPKSFMDHPSNR-UHFFFAOYAN
Related Products
- DL-3-Aminoisobutyric acid
- 105706-75-0
- 105706-76-1
- 10570-67-9
- 105707-24-2
- 1057-07-4
- 1057077-52-7
- 105708-74-5
- 1057097-72-9
- 10572-16-4
- 1057216-55-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View