-
Name
Di-n-butyl ether
- EINECS 205-575-3
- CAS No. 142-96-1
- Article Data153
- CAS DataBase
- Density 0.78 g/cm3
- Solubility water: 0.03 g/100 mL (20 °C)
- Melting Point -98 °C(lit.)
- Formula C8H18O
- Boiling Point 142.1 °C at 760 mmHg
- Molecular Weight 130.23
- Flash Point 25 °C
- Transport Information UN 1149 3/PG 3
- Appearance colourless liquid with ether-like odour
- Safety 61
- Risk Codes 10-36/37/38-52/53
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Butyl ether(8CI);1,1'-Oxybisbutane;Butyl oxide;Dibutyl ether;Dibutyloxide;NSC 8459;n-Butyl ether;
- PSA 9.23000
- LogP 2.60320
Synthetic route

| Conditions | Yield |
|---|---|
| In water | 97% |


| Conditions | Yield |
|---|---|
| In water; butan-1-ol | 96.1% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tetrabutylphosphonium ion; silica gel at 170℃; under 760 Torr; | A 95% B 4 % Chromat. |

| Conditions | Yield |
|---|---|
| With hydrogen; Pd-C | 95% |
| With triethylsilane; silver hexafluoroantimonate at 20℃; for 1.5h; Green chemistry; | 80% |
| With triethylsilane; 1-diphenylphosphino-8-triphenylstibonium-naphthalene triflate In dichloromethane at 20℃; for 0.25h; Catalytic behavior; Reagent/catalyst; Time; | 100 %Spectr. |
| Stage #1: butyraldehyde With C18H25IrN4O(2+)*2CF3O3S(1-) In dichloromethane at 20℃; for 0.0833333h; Stage #2: With phenylsilane In dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; carbon tetrabromide at 200℃; for 10h; Inert atmosphere; Sealed tube; | 92% |
| With sulfuric acid; silica gel In hexane for 1h; Heating; | 80% |
| With phosphotungstic acid In toluene at 200℃; under 22502.3 Torr; for 3h; Reagent/catalyst; Autoclave; Inert atmosphere; | 73.2% |
-
-
71-36-3
butan-1-ol

-
A
-
126-73-8
phosphoric acid tributyl ester

-
B
-
109-69-3
n-Butyl chloride

-
C
-
142-96-1
dibutyl ether

| Conditions | Yield |
|---|---|
| With oxygen; phosphan; copper(l) chloride; copper dichloride at 24.9℃; Kinetics; Product distribution; Mechanism; ΔE(excit.), ΔS(excit.); other reagents, other catalysts; | A 90% B n/a C n/a |
| With oxygen; phosphan; copper(l) chloride; copper dichloride at 24.9℃; | A 90% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With Cp*Ir(Cl)2(nBu2Im); silver trifluoromethanesulfonate at 130℃; for 2h; | A n/a B 90% |

-
-
57-48-7
D-Fructose

-
-
71-36-3
butan-1-ol

-
A
-
1917-68-6
5-(butoxymethyl)furan-2-carbaldehyde

-
B
-
142-96-1
dibutyl ether

-
C
-
592-84-7
n-butyl formate

-
D
-
2052-15-5
butyl levulinate

| Conditions | Yield |
|---|---|
| With poly(p-styrenesulfonic acid)-grafted carbon nanotubes at 120℃; for 24h; Sealed tube; Green chemistry; chemoselective reaction; | A n/a B n/a C n/a D 78% |

-
-
98-00-0
(2-furyl)methyl alcohol

-
-
71-36-3
butan-1-ol

-
A
-
142-96-1
dibutyl ether

-
B
-
2052-15-5
butyl levulinate

| Conditions | Yield |
|---|---|
| With AQUIVION P87S perfluorosulfonic acid resin at 120℃; for 5h; Reagent/catalyst; Sealed tube; | A n/a B 76% |


| Conditions | Yield |
|---|---|
| With potassium carbonate at 100℃; for 20h; | 60% |

| Conditions | Yield |
|---|---|
| 3-methyl-1-(4-sulfobutyl)imidazol-3-ium bis((trifluoromethyl)sulfonyl)azanide at 250℃; for 0.5h; Product distribution / selectivity; Irradiation; | A n/a B n/a C n/a D 57% |
| With sulfated ZrO2 at 150℃; under 760.051 Torr; Kinetics; Temperature; |

-
-
98-00-0
(2-furyl)methyl alcohol

-
-
71-36-3
butan-1-ol

-
A
-
56920-82-2
furfuryl alcohol butyl ether

-
B
-
142-96-1
dibutyl ether

-
C
-
2052-15-5
butyl levulinate

| Conditions | Yield |
|---|---|
| With AQUIVION P87S perfluorosulfonic acid resin at 120℃; for 2h; Catalytic behavior; Reagent/catalyst; Sealed tube; | A 9% B n/a C 56% D n/a |

-
-
100-51-6
benzyl alcohol

-
-
71-36-3
butan-1-ol

-
A
-
142-96-1
dibutyl ether

-
B
-
103-50-4
dibenzyl ether

-
C
-
588-67-0
benzyl 1-butyl ether

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; carbon tetrabromide at 150℃; for 8h; Inert atmosphere; Sealed tube; | A 25% B 40% C 55% |
| With 5 wt percent copper(II) bromide supported on zeolite HY at 150℃; under 760.051 Torr; for 8h; | A n/a B n/a C 45% |


| Conditions | Yield |
|---|---|
| With hydrogen; aluminum oxide; ruthenium-copper at 150℃; | A 52% B 48% |
| With hydrogen; aluminum oxide; ruthenium-copper at 150℃; Mechanism; Product distribution; var. catalysts; |
-
-
67-56-1
methanol

-
-
78-83-1
2-methyl-propan-1-ol

-
A
-
1634-04-4
tert-butyl methyl ether

-
B
-
625-44-5
isobutyl methyl ether

-
C
-
142-96-1
dibutyl ether

| Conditions | Yield |
|---|---|
| With chromium(III) oxide Heating; | A 50% B 1.7 g C 1.3 g |


| Conditions | Yield |
|---|---|
| In water | 97% |


| Conditions | Yield |
|---|---|
| In water; butan-1-ol | 96.1% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tetrabutylphosphonium ion; silica gel at 170℃; under 760 Torr; | A 95% B 4 % Chromat. |

| Conditions | Yield |
|---|---|
| With hydrogen; Pd-C | 95% |
| With triethylsilane; silver hexafluoroantimonate at 20℃; for 1.5h; Green chemistry; | 80% |
| With triethylsilane; 1-diphenylphosphino-8-triphenylstibonium-naphthalene triflate In dichloromethane at 20℃; for 0.25h; Catalytic behavior; Reagent/catalyst; Time; | 100 %Spectr. |
| Stage #1: butyraldehyde With C18H25IrN4O(2+)*2CF3O3S(1-) In dichloromethane at 20℃; for 0.0833333h; Stage #2: With phenylsilane In dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; carbon tetrabromide at 200℃; for 10h; Inert atmosphere; Sealed tube; | 92% |
| With sulfuric acid; silica gel In hexane for 1h; Heating; | 80% |
| With phosphotungstic acid In toluene at 200℃; under 22502.3 Torr; for 3h; Reagent/catalyst; Autoclave; Inert atmosphere; | 73.2% |
-
-
71-36-3
butan-1-ol

-
A
-
126-73-8
phosphoric acid tributyl ester

-
B
-
109-69-3
n-Butyl chloride

-
C
-
142-96-1
dibutyl ether

| Conditions | Yield |
|---|---|
| With oxygen; phosphan; copper(l) chloride; copper dichloride at 24.9℃; Kinetics; Product distribution; Mechanism; ΔE(excit.), ΔS(excit.); other reagents, other catalysts; | A 90% B n/a C n/a |
| With oxygen; phosphan; copper(l) chloride; copper dichloride at 24.9℃; | A 90% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With Cp*Ir(Cl)2(nBu2Im); silver trifluoromethanesulfonate at 130℃; for 2h; | A n/a B 90% |

-
-
57-48-7
D-Fructose

-
-
71-36-3
butan-1-ol

-
A
-
1917-68-6
5-(butoxymethyl)furan-2-carbaldehyde

-
B
-
142-96-1
dibutyl ether

-
C
-
592-84-7
n-butyl formate

-
D
-
2052-15-5
butyl levulinate

| Conditions | Yield |
|---|---|
| With poly(p-styrenesulfonic acid)-grafted carbon nanotubes at 120℃; for 24h; Sealed tube; Green chemistry; chemoselective reaction; | A n/a B n/a C n/a D 78% |

-
-
98-00-0
(2-furyl)methyl alcohol

-
-
71-36-3
butan-1-ol

-
A
-
142-96-1
dibutyl ether

-
B
-
2052-15-5
butyl levulinate

| Conditions | Yield |
|---|---|
| With AQUIVION P87S perfluorosulfonic acid resin at 120℃; for 5h; Reagent/catalyst; Sealed tube; | A n/a B 76% |


| Conditions | Yield |
|---|---|
| With potassium carbonate at 100℃; for 20h; | 60% |
-
-
71-36-3
butan-1-ol

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
142-96-1
dibutyl ether

| Conditions | Yield |
|---|---|
| 3-methyl-1-(4-sulfobutyl)imidazol-3-ium bis((trifluoromethyl)sulfonyl)azanide at 250℃; for 0.5h; Product distribution / selectivity; Irradiation; | A n/a B n/a C n/a D 57% |
| With sulfated ZrO2 at 150℃; under 760.051 Torr; Kinetics; Temperature; |

-
-
98-00-0
(2-furyl)methyl alcohol

-
-
71-36-3
butan-1-ol

-
A
-
56920-82-2
furfuryl alcohol butyl ether

-
B
-
142-96-1
dibutyl ether

-
C
-
2052-15-5
butyl levulinate

| Conditions | Yield |
|---|---|
| With AQUIVION P87S perfluorosulfonic acid resin at 120℃; for 2h; Catalytic behavior; Reagent/catalyst; Sealed tube; | A 9% B n/a C 56% D n/a |

-
-
100-51-6
benzyl alcohol

-
-
71-36-3
butan-1-ol

-
A
-
142-96-1
dibutyl ether

-
B
-
103-50-4
dibenzyl ether

-
C
-
588-67-0
benzyl 1-butyl ether

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; carbon tetrabromide at 150℃; for 8h; Inert atmosphere; Sealed tube; | A 25% B 40% C 55% |
| With 5 wt percent copper(II) bromide supported on zeolite HY at 150℃; under 760.051 Torr; for 8h; | A n/a B n/a C 45% |


| Conditions | Yield |
|---|---|
| With hydrogen; aluminum oxide; ruthenium-copper at 150℃; | A 52% B 48% |
| With hydrogen; aluminum oxide; ruthenium-copper at 150℃; Mechanism; Product distribution; var. catalysts; |
-
-
67-56-1
methanol

-
-
78-83-1
2-methyl-propan-1-ol

-
A
-
1634-04-4
tert-butyl methyl ether

-
B
-
625-44-5
isobutyl methyl ether

-
C
-
142-96-1
dibutyl ether

| Conditions | Yield |
|---|---|
| With chromium(III) oxide Heating; | A 50% B 1.7 g C 1.3 g |


| Conditions | Yield |
|---|---|
| With sulfuric acid at 0 - 200℃; Reagent/catalyst; Temperature; Autoclave; | A n/a B 50% |

-
-
64-17-5
ethanol

-
-
67-64-1
acetone

-
-
71-36-3
butan-1-ol

-
A
-
110-43-0
n-pentyl methyl ketone

-
B
-
142-96-1
dibutyl ether

-
C
-
107-87-9
2-Pentanone

| Conditions | Yield |
|---|---|
| With Pd modified CsY zeolite at 215℃; | A 47% B 19 mg C 7.3% |


| Conditions | Yield |
|---|---|
| 3-methyl-1-(4-sulfobutyl)imidazol-3-ium bis((trifluoromethyl)sulfonyl)azanide at 350℃; Product distribution / selectivity; | A n/a B n/a C 45% |
| monoaluminum phosphate at 299.9℃; | A 7.8% B 2.4% C 32.9% |

-
-
64-17-5
ethanol

-
-
67-64-1
acetone

-
-
71-36-3
butan-1-ol

-
A
-
110-43-0
n-pentyl methyl ketone

-
B
-
142-96-1
dibutyl ether

| Conditions | Yield |
|---|---|
| With Pd modified CsY zeolite at 240℃; | A 43.4% B 156 mg |


| Conditions | Yield |
|---|---|
| With magnesium hydride; zirconium(IV) chloride In tetrahydrofuran at 66 - 80℃; | A n/a B 34% |

| Conditions | Yield |
|---|---|
| With Amberlyst 36 at 150℃; under 30.003 Torr; for 7h; Catalytic behavior; | A 20.1% B n/a |
| DELOXAN ASP In carbon dioxide at 200℃; under 200 Torr; | A 33 % Turnov. B 1 % Turnov. |

| Conditions | Yield |
|---|---|
| With hydrogen; Cobalt rhodium; iodine at 200℃; under 420 Torr; for 2h; Product distribution; other promoter, other pressure; | A 16% B 19% |

-
-
71-36-3
butan-1-ol

-
A
-
624-64-6
trans-2-Butene

-
B
-
142-96-1
dibutyl ether

-
C
-
999-65-5
1-(1-methylpropoxy)butane

| Conditions | Yield |
|---|---|
| With Amberlyst 35 at 150℃; under 30.003 Torr; for 7h; Catalytic behavior; | A n/a B 16.6% C n/a |

-
-
71-36-3
butan-1-ol

-
A
-
106-98-9
1-butylene

-
B
-
187737-37-7
propene

-
C
-
142-96-1
dibutyl ether

-
D
-
123-72-8
butyraldehyde

| Conditions | Yield |
|---|---|
| γ-Al2O3 at 299.9℃; Further byproducts given; | A 4.3% B 2.1% C 11.5% D 2.4% |

-
-
71-36-3
butan-1-ol

-
A
-
106-98-9
1-butylene

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
142-96-1
dibutyl ether

-
D
-
123-72-8
butyraldehyde

| Conditions | Yield |
|---|---|
| monoaluminum phosphate at 299.9℃; Further byproducts given; | A 1.3% B 0.4% C 5.3% D 1.9% |

-
-
71-36-3
butan-1-ol

-
A
-
106-98-9
1-butylene

-
B
-
142-96-1
dibutyl ether

-
C
-
123-72-8
butyraldehyde

-
D
-
105-54-4
butanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| monoaluminum phosphate at 299.9℃; Further byproducts given; | A 1.3% B 5.3% C 1.9% D 1.5% |


| Conditions | Yield |
|---|---|
| With ammonia under 7600 Torr; |

| Conditions | Yield |
|---|---|
| With ammonia |

| Conditions | Yield |
|---|---|
| With diethyl ether |

| Conditions | Yield |
|---|---|
| With Selectfluor; trifluoroacetic acid In acetonitrile at 25℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 99% |
| With sodium persulfate; [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; trifluoroacetic acid In water at 23℃; for 4h; Minisci Aromatic Substitution; Inert atmosphere; Irradiation; regioselective reaction; | 88% |
| With dipotassium peroxodisulfate; trifluoroacetic acid In water; acetonitrile for 2h; Solvent; Minisci Aromatic Substitution; Reflux; | 68% |

| Conditions | Yield |
|---|---|
| With palladium diacetate at 100℃; for 2h; Microwave irradiation; | 96.1% |
| With molybdenum(V) chloride In dichloromethane at 80℃; for 3h; | 75% |
| With molybdenum(V) chloride In 1,2-dichloro-ethane at 80℃; for 3h; | 75% |
-
-
142-96-1
dibutyl ether

-
-
652-03-9
3,4,5,6-tetrafluorophthalic acid

-
-
1201-31-6
2,3,4,5-tetrafluorobenzoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; dimethyl sulfoxide; triethylamine In water; toluene | 94% |
-
-
957188-75-9
[N-(trifluoromethylsulfonyl)imino][4-(trifluoromethyl)phenyl]-λ3-bromane

-
-
142-96-1
dibutyl ether

-
-
1378022-03-7
N-(1-butoxybutyl)trifluoromethanesulfonamide

| Conditions | Yield |
|---|---|
| at 20℃; for 2h; Inert atmosphere; regioselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With Benzoylformic acid at 20℃; Sealed tube; Irradiation; diastereoselective reaction; | 93% |
-
-
142-96-1
dibutyl ether

-
-
18039-42-4
5-phenyl-2H-1,2,3,4-tetrazole

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In water at 80℃; for 12h; | 90% |
| With tert.-butylhydroperoxide; iron(III) chloride hexahydrate In water; 1,2-dichloro-ethane at 90℃; for 12h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With water; tetraphenylphosphonium bromide; potassium hydroxide at 60℃; for 24h; Inert atmosphere; Darkness; | A 89% B 74% |
| With water; tetraphenylphosphonium bromide; potassium hydroxide at 60℃; for 24h; Inert atmosphere; Darkness; | A 56 %Chromat. B 83% |


| Conditions | Yield |
|---|---|
| With hexaethylbisphosphonium bistriiodide Heating; | 88% |
| With phosphoric acid; potassium iodide |

| Conditions | Yield |
|---|---|
| In dibutyl ether; toluene byproducts: LiBr, LiOMe; (N2); Schlenk technique; soln. of PhLi in Bu2O was dild. with toluene and added dropwise with stirring to soln. of Fe complex in toluene at -78°C; slowly warmed to room temp.; stirred overnight; filtered; stored at -35°C for 3 d; elem. anal.; | 87% |

-
-
85990-32-5
(5,10,15,20-tetra(2,4,6-trimethylphenyl)porphyrinato)rhodium(III) iodide

-
-
142-96-1
dibutyl ether

-
-
940002-36-8
propyl(5,10,15,20-tetramesitylporphyrinato)rhodium(III)

| Conditions | Yield |
|---|---|
| With KOH In dibutyl ether reaction of rhodium compd. with n-butyl ether in presence of 10 equiv. of KOH at 100°C for 1 d under N2; | 86% |
| With KOH In dibutyl ether reaction of rhodium compd. with n-butyl ether in presence of 10 equiv. of KOH at 80°C for 1 d under N2; | 53% |
| With KOH In dibutyl ether reaction of rhodium compd. with n-butyl ether in presence of 20 equiv. of KOH at 40°C for 1 d under N2; | 35% |

| Conditions | Yield |
|---|---|
| With water; tetraphenylphosphonium bromide; potassium hydroxide at 100℃; for 24h; Inert atmosphere; Darkness; | 86% |

| Conditions | Yield |
|---|---|
| FeCl3-Montmorillonite K-10 at 70℃; for 24h; | 83% |
| With thallium(III) nitrate Ambient temperature; | 57% |
| With sulfuric acid |
-
-
142-96-1
dibutyl ether

-
-
201230-82-2
carbon monoxide

-
-
459-46-1
4-Fluorobenzyl bromide

-
A
-
109-65-9
1-bromo-butane

-
B
-
104548-37-0
(4-Fluoro-phenyl)-acetic acid butyl ester

| Conditions | Yield |
|---|---|
| 1,5-hexadienerhodium(I)-chloride dimer; potassium iodide at 75 - 90℃; under 735.5 Torr; overnight or in n-heptane; | A n/a B 83% |

-
-
121393-39-3
5,10,15,20-tetra(2,4,6-trimethylphenyl)porphyrinate rhodium(II)

-
-
142-96-1
dibutyl ether

-
-
940002-36-8
propyl(5,10,15,20-tetramesitylporphyrinato)rhodium(III)

| Conditions | Yield |
|---|---|
| With PPh3; KOH; Ph4PBr In dibutyl ether; water byproducts: HCO2Bu; under N2; in the dark; Rh compd. reacted with n-butyl ether in presence of PPh3 (1 equiv.), KOH (10 equiv.), H2O (50 equiv.) and Ph4PBr (0.1 equiv.) at 25°C for 10 min; | 83% |
| In dibutyl ether reaction of rhodium compd. with dibutyl ether at 100°C for 1 d; | 37% |
-
-
121393-39-3
5,10,15,20-tetra(2,4,6-trimethylphenyl)porphyrinate rhodium(II)

-
-
142-96-1
dibutyl ether

-
A
-
592-84-7
n-butyl formate

-
-
940002-36-8
propyl(5,10,15,20-tetramesitylporphyrinato)rhodium(III)

| Conditions | Yield |
|---|---|
| With water; tetraphenylphosphonium bromide; triphenylphosphine; potassium hydroxide at 25℃; Inert atmosphere; Darkness; regioselective reaction; | A 48 %Chromat. B 83% |

Di-n-butyl ether Consensus Reports
Di-n-butyl ether Standards and Recommendations
Di-n-butyl ether Specification
The n-Butyl ether is an organic compound with the formula C8H18O. The IUPAC name of this chemical is 1-butoxybutane. With the CAS registry number 142-96-1, it is also named as 1,1'-Oxybisbutane. The product's classification codes are Human Data; Skin / Eye Irritant. Besides, it should be stored in a closed cool and dry place. It is used as reagents of determination of bismuth, solvents, extractant and in organic synthesis.
Physical properties about n-Butyl ether are: (1)ACD/LogP: 3.11; (2)ACD/LogD (pH 5.5): 3.11; (3)ACD/LogD (pH 7.4): 3.11; (4)ACD/BCF (pH 5.5): 135.23; (5)ACD/BCF (pH 7.4): 135.23; (6)ACD/KOC (pH 5.5): 1166.88; (7)ACD/KOC (pH 7.4): 1166.88; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 6; (10)Polar Surface Area: 9.23 Å2; (11)Index of Refraction: 1.404; (12)Molar Refractivity: 40.85 cm3; (13)Molar Volume: 166.9 cm3; (14)Polarizability: 16.19×10-24cm3; (15)Surface Tension: 24.1 dyne/cm; (16)Density: 0.78 g/cm3; (17)Flash Point: 25 °C; (18)Enthalpy of Vaporization: 36.36 kJ/mol; (19)Boiling Point: 142.1 °C at 760 mmHg; (20)Vapour Pressure: 7.1 mmHg at 25°C.
Preparation: this chemical can be prepared by butan-1-ol and n-Butyldiphenylsulfoniumperchlorat. This reaction will need reagent K2CO3. The reaction time is 20 min. The yield is about 60%.

Uses of n-Butyl ether: it can be used to produce 1-iodo-butane. It will need reagent phosphoric acid and potassium iodide.
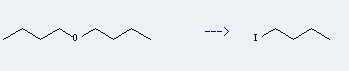
When you are using this chemical, please be cautious about it as the following:
It is flammable and harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. Besides, this chemical is irritating to eyes, respiratory system and skin. When you are using it, avoid release to the environment. Refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: O(CCCC)CCCC
(2)InChI: InChI=1/C8H18O/c1-3-5-7-9-8-6-4-2/h3-8H2,1-2H3
(3)InChIKey: DURPTKYDGMDSBL-UHFFFAOYAH
(4)Std. InChI: InChI=1S/C8H18O/c1-3-5-7-9-8-6-4-2/h3-8H2,1-2H3
(5)Std. InChIKey: DURPTKYDGMDSBL-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | TCLo | inhalation | 200ppm (200ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE | Journal of Industrial Hygiene and Toxicology. Vol. 28, Pg. 262, 1946. |
| mouse | LC50 | inhalation | 169gm/m3/15M (169000mg/m3) | BEHAVIORAL: GENERAL ANESTHETIC | Anesthesiology. Vol. 11, Pg. 455, 1950. |
| mouse | LDLo | intravenous | 258mg/kg (258mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Acta Pharmacologica et Toxicologica. Vol. 37, Pg. 56, 1975. |
| rabbit | LD50 | skin | 10mL/kg (10mL/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. | |
| rat | LCLo | inhalation | 4000ppm/4H (4000ppm) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. | |
| rat | LD50 | oral | 7400mg/kg (7400mg/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. |
Related Products
- Di-n-Butyl Dineodecanoate Tin
- Di-n-butyl ether
- Di-n-butyl(dibutyryloxy)stannane
- Di-N-butylammonium hexafluoroarsenate
- Di-n-butylbis(dodecylthio)tin
- Di-n-butyltin bismethanesulfonate
- Di-n-butyltin di(monononyl)maleate
- 14296-92-5
- 14297-34-8
- 142975-31-3
- 14297-81-5
- 14297-93-9
- 142979-40-6
- 142979-68-8
- 14298-19-2
- 142982-20-5
- 14298-31-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View