-
Name
N,N-Diallylamine
- EINECS 204-671-2
- CAS No. 124-02-7
- Article Data31
- CAS DataBase
- Density 0.767 g/cm3
- Solubility 8.6 g/100mL
- Melting Point -88 °C
- Formula C6H11N
- Boiling Point 115.3 °C at 760 mmHg
- Molecular Weight 97.16
- Flash Point 15.6 °C
- Transport Information UN 2359 3/PG 2
- Appearance clear colourless to yellow liquid
- Safety 16-26-36/37/39-45-28
- Risk Codes 11-20/22-24-34
-
Molecular Structure
-
Hazard Symbols
 T,
T, F
F
- Synonyms 2-propen-1-amine, N-2-propen-1-yl-;N-(prop-2-en-1-yl)prop-2-en-1-amine;N-Allyl-2-propen-1-amine;N-Allylprop-2-en-1-amine;Diallylamine;
- PSA 12.03000
- LogP 1.33890
Synthetic route

-
A
-
34911-33-6
5,7-Dimethoxy-2-phenylbenzofuran

-
B
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| In benzene Irradiation; | A 96% B 56% |

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran for 4h; Ambient temperature; | 90% |
-
-
109-73-9
N-butylamine

-
-
107-18-6
allyl alcohol

-
A
-
4538-09-4
N-allyl-N-butylamine

-
B
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With ammonium hexafluorophosphate; [Pt(η3-C3H5)(DPP-Xantphos)]PF6 In toluene; acetonitrile at 30℃; for 44h; Inert atmosphere; | A 85% B 4% |

-
-
111-26-2
hexan-1-amine

-
-
107-18-6
allyl alcohol

-
A
-
22774-71-6
N-allylhexan-1-amine

-
B
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With ammonium hexafluorophosphate; [Pt(η3-C3H5)(DPP-Xantphos)]PF6 In toluene; acetonitrile at 50℃; for 8h; Inert atmosphere; | A 81% B 7% |


| Conditions | Yield |
|---|---|
| With ammonium hexafluorophosphate; [Pt(η3-C3H5)(DPP-Xantphos)]PF6 In toluene; acetonitrile at 30℃; for 20h; Inert atmosphere; | A 80% B 7% |


-
-
594-19-4
tert.-butyl lithium

-
A
-
7283-47-8
(4,4-dimethylpent-1-en-2-yl)benzene

-
B
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| Stage #1: N,N-diallyl-2-phenyl-2-propen-1-amine; tert.-butyl lithium In tetrahydrofuran at -78 - 0℃; Stage #2: With water In tetrahydrofuran | A n/a B 79% |


-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| Stage #1: N,N-diallyltritylamine With naphthalene; lithium In tetrahydrofuran at 0℃; for 6h; Stage #2: With water In tetrahydrofuran at 0 - 20℃; | 62% |

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With sulfuric acid In water at 30℃; |
-
-
463-49-0
1,2-propanediene

-
-
107-11-9
1-amino-2-propene

-
A
-
102-70-5
Triallylamine

-
B
-
86660-16-4
N-allyl(2,3-dimethylene)butylamine

-
C
-
86660-17-5
Diallyl-(3-methyl-2-methylene-but-3-enyl)-amine

-
E
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| sodium tetrahydroborate; triphenylphosphine; palladium dichloride at 150℃; for 1h; Product distribution; other temperatures, times; reaction with diallyl amine and N-allyl(2,3-dimethylene)butylamine; telomerization of propadiene with allyl amines; |

-
-
463-49-0
1,2-propanediene

-
-
107-11-9
1-amino-2-propene

-
A
-
86660-16-4
N-allyl(2,3-dimethylene)butylamine

-
B
-
86660-17-5
Diallyl-(3-methyl-2-methylene-but-3-enyl)-amine

-
D
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| sodium tetrahydroborate; triphenylphosphine; palladium dichloride at 130℃; for 3h; |

-
-
463-49-0
1,2-propanediene

-
A
-
86660-17-5
Diallyl-(3-methyl-2-methylene-but-3-enyl)-amine

-
B
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| sodium tetrahydroborate; triphenylphosphine; palladium dichloride at 130℃; for 6h; |

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) CH2Cl2, -20 deg C, 10 min, 2.) room temperature, 30 min, 3.) 70 deg C, 1 h; Yield given. Multistep reaction; |
-
-
7664-41-7
ammonia

-
-
107-05-1
3-chloroprop-1-ene

-
A
-
102-70-5
Triallylamine

-
B
-
107-11-9
1-amino-2-propene

-
C
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| at 97 - 105℃; unter Druck; |


| Conditions | Yield |
|---|---|
| With ammonia at 97 - 105℃; under 5884.06 - 11032.6 Torr; | |
| With ammonia at 97 - 105℃; under 5884.06 - 11032.6 Torr; |

| Conditions | Yield |
|---|---|
| With ammonia at 10℃; |


| Conditions | Yield |
|---|---|
| Hydrolysis; |

| Conditions | Yield |
|---|---|
| Hydrolysis; |

| Conditions | Yield |
|---|---|
| With alkaline potassium hexacyanoferrate(III) In ethanol at 25℃; Mechanism; Rate constant; Kinetics; variations in the ionic strength of the reaction medium (cations and anions), varying amount of solvent; ΔG(excit.), ΔH(excit.), ΔS(excit.); |
-
-
25070-76-2
benzyl diallylcarbamate

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With naphthalen-1-yl-lithium In tetrahydrofuran at 0℃; for 1h; | 62 % Chromat. |
-
-
107-05-1
3-chloroprop-1-ene

-
A
-
102-70-5
Triallylamine

-
B
-
107-11-9
1-amino-2-propene

-
C
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloroprop-1-ene With formaldehyd; ammonia In methanol; water at 40 - 70℃; for 6h; Stage #2: With hydroxyammonium sulfate; sulfuric acid In methanol; water at 40℃; for 0.5h; pH=0.8; Stage #3: With sodium hydroxide In methanol; water pH=12; Product distribution / selectivity; | |
| Stage #1: 3-chloroprop-1-ene With ammonia In methanol; water at 40 - 70℃; for 6h; Stage #2: With hydroxyammonium sulfate; sulfuric acid In methanol; water at 40℃; for 0.5h; pH=0.8; Stage #3: With sodium hydroxide In methanol; water pH=12; Product distribution / selectivity; | |
| With ammonium hydroxide at 35℃; for 0.666667h; Temperature; |

-
-
110-89-4
piperidine

-
-
26256-86-0
C13H23NO

-
A
-
18494-53-6
1-(piperidin-1-yl)heptan-1-one

-
B
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With Al2(NMe2)6 In toluene at 90℃; for 16h; Equilibrium constant; |


| Conditions | Yield |
|---|---|
| With Al2(NMe2)6 In toluene at 90℃; for 16h; Equilibrium constant; |

-
-
463-49-0
1,2-propanediene

-
A
-
102-70-5
Triallylamine

-
B
-
107-11-9
1-amino-2-propene

-
C
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With AuC27H42N2(1+)*B(C6F5)4(1-)=(AuC27H42N2)(B(C6F5)4); ammonia In benzene-d6 at -60 - 165℃; Inert atmosphere; | |
| With ammonia; AuC27H42N2(1+)*B(C6F5)4(1-)=(AuC27H42N2)(B(C6F5)4) In benzyl methyl ether; benzene-d6 at -60 - 175℃; Conversion of starting material; |


| Conditions | Yield |
|---|---|
| With AuC27H42N2(1+)*B(C6F5)4(1-)=(AuC27H42N2)(B(C6F5)4); ammonia In benzene-d6 at -60 - 175℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With rhodium(III) 5,10,15,20-tetra(p-sulfonatophenyl)porphyrin; oxygen; trifluoroacetic acid In water at 100℃; under 760.051 Torr; for 2h; |

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| Stage #1: diphenyl diallylphosphoramidate With water; monoammonium para-toluenesulfonate; o-phthalic dicarboxaldehyde In acetonitrile at 90℃; for 24h; Sealed tube; Stage #2: With sodium hydroxide In water pH=8 - 9; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| In ethanol for 24h; Heating; | 100% |
| In ethanol at 95℃; Sealed tube; | 100% |
| With triethylaluminum 1.) toluene, CH2Cl2, 25-30 deg C, 30 min, 2.) room temperature; Yield given. Multistep reaction; |
-
-
3376-23-6, 7372-59-0, 59862-60-1
C-phenyl-N-methylnitrone

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| In chloroform at 60℃; for 72h; | 100% |
| In chloroform at 60℃; for 72h; Cyclization; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: diallylamine; ethyl trans-3-phenyl-1-oxirane-2-carboxylate In ethanol for 12h; Ring cleavage; addition; Heating; Stage #2: With methanesulfonyl chloride; triethylamine In dichloromethane at 0 - 20℃; for 3h; Rearrangement; chlorination; | 100% |
-
-
21040-45-9
Cinnamyl acetate

-
-
124-02-7
diallylamine

-
-
155687-70-0
(E)-N,N-diallyl-3-phenylprop-2-en-1-amine

| Conditions | Yield |
|---|---|
| bis(η3-allyl-μ-chloropalladium(II)); (1RS,2RS,3SR,4SR)-1,2,3,4-tetrakis((diphenylphosphanyl)methyl)cyclopentane In water at 25℃; for 40h; | 100% |
| bis(η3-allyl-μ-chloropalladium(II)); Tedicyp In water at 25℃; for 40h; | 99% |
-
-
925211-06-9, 925211-07-0
bicyclo(3.2.0)hept-2-en-6-one

-
-
126421-91-8
5-phenyl-[1,2,4]triazine-3-carboxylic acid ethyl ester

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In chloroform for 21h; Heating; | 100% |
| With 4 A molecular sieve In chloroform for 21h; Heating; | 100% |

-
-
1191-95-3
cyclobutanone

-
-
126421-91-8
5-phenyl-[1,2,4]triazine-3-carboxylic acid ethyl ester

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In chloroform for 22h; Heating; | 100% |
| With 4 A molecular sieve In chloroform for 22h; Heating; | 99% |

-
-
19545-26-7
wortmannin

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; | 100% |
| In dichloromethane at 20℃; for 1h; | 68% |
-
-
124-02-7
diallylamine

-
-
252577-46-1
(1R,2S,4S)-(+)-(N-bromoacetyl)bornane-10,2-sultam

-
-
844498-35-7
(1R,2S)-N-[2'-(diallylamino)acetyl]bornane-10,2-sultam

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; | 100% |
-
-
15761-38-3
L-N-Boc-Ala

-
-
124-02-7
diallylamine

-
-
950608-92-1
(S)-tert-butyl 1-(diallylamino)-1-oxopropan-2-ylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: L-N-Boc-Ala With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 0.5h; Inert atmosphere; Stage #2: diallylamine In dichloromethane at 20℃; for 12h; Inert atmosphere; | 100% |
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 12h; |
-
-
884540-65-2
17-formylwortmannin

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 1.5h; | 100% |
-
-
124-02-7
diallylamine

-
-
76-02-8
Trichloroacetyl chloride

-
-
39089-57-1
N,N-diallyl-α,α,α-trichloroacetamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 2h; | 100% |
| With pyridine In dichloromethane at 20℃; for 2h; | 94% |
-
-
1191-99-7
2,3-dihydro-2H-furan

-
-
75-15-0
carbon disulfide

-
-
124-02-7
diallylamine

-
-
1239488-67-5
tetrahydrofuran-2-yl diallylcarbamodithioate

| Conditions | Yield |
|---|---|
| In water at 20℃; for 16h; regiospecific reaction; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: oxalyl dichloride; Benzoylformic acid With copper diacetate; N,N-dimethyl-formamide In dichloromethane at 20℃; for 2h; Stage #2: diallylamine With triethylamine In dichloromethane | 100% |


| Conditions | Yield |
|---|---|
| With dmap; triethylamine In 1,2-dichloro-ethane Reflux; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 14h; Inert atmosphere; | 100% |
-
-
23095-16-1
2-bromo-4-methoxybenzenesulfonyl chloride

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 15h; | 100% |
-
-
179251-57-1
2-bromo-5-methoxybenzenesulfonyl chloride

-
-
124-02-7
diallylamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 15h; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 0.166667h; | 99% |
| With 1,6-anhydro-3,4-dideoxy-β-D-glycero-hexopyranos-2-ulose at 0 - 20℃; for 1h; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 0℃; for 0.5h; | 99% |
| With trimethylamine In ethyl acetate at 23℃; for 18h; Cooling with ice; | 75% |
| With triethylamine In dichloromethane for 24h; Ambient temperature; | 71% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 0℃; Michael addition; | 99% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at -15℃; for 0.166667h; | 95% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at -15℃; for 0.666667h; |

| Conditions | Yield |
|---|---|
| In methanol at 0 - 20℃; for 1h; | 99% |
| With iodine at 20℃; for 0.5h; | 95% |
| In cyclohexane at 20℃; for 20h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzoyl chloride With pyridine; Merrifield's resin-bound 6-methyl-2-thiouracil In dichloromethane at 80℃; for 0.0833333h; microwave irradiation; Stage #2: diallylamine In dichloromethane at 80℃; for 0.0833333h; microwave irradiation; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 97% |
| With triethylamine In dichloromethane at 0 - 20℃; | 89% |
-
-
22921-68-2
2-bromo-5-methoxy-benzoic acid

-
-
124-02-7
diallylamine

-
-
851297-49-9
N,N-diallyl-2-bromo-5-methoxy-benzamide

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With 4C14H11NO3(2-)*2Ni(2+)*2Dy(3+)*2Cl(1-)*2C2H3N In acetonitrile at 20℃; for 24h; Reagent/catalyst; Molecular sieve; | 99% |
| In water at 60℃; for 0.0833333h; Microwave irradiation; | 93% |
| With 4 A molecular sieve; dysprosium(III) trifluoromethanesulfonate In acetonitrile at 20℃; for 16h; | 82% |
-
-
37517-81-0
3-chloro-3-oxopropanoic acid methyl ester

-
-
124-02-7
diallylamine

-
-
1193366-04-9
methyl 3-(N,N-diallylamino)-3-oxopropanoate

| Conditions | Yield |
|---|---|
| In dichloromethane at 10 - 20℃; | 99% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
124-02-7
diallylamine

-
-
1211531-07-5
tert-butyl di(but-3-en-1-yl)carbamate

| Conditions | Yield |
|---|---|
| In methanol at 0 - 20℃; | 99% |
-
-
87751-69-7
rac-(E)-1,3-diphenyl-3-acetoxy-prop-1-ene

-
-
124-02-7
diallylamine

-
-
1202053-74-4
(S,E)-N,N-diallyl-(1,3-diphenyl-2-propenyl)amine

| Conditions | Yield |
|---|---|
| With C32H31NO2PPd(1+)*F6P(1-); potassium acetate; N,O-bis-(trimethylsilyl)-acetamide at 20℃; for 4h; Inert atmosphere; Neat (no solvent); enantioselective reaction; | 99% |
| With N,O-bis-(trimethylsilyl)-acetamide; bis(η3-allyl-μ-chloropalladium(II)); 1-benzyl-5-(((4R,5R)-2-(2-(diphenyl-phosphino)phenyl)-4,5-diphenyl-4,5-dihydro-1H-imidazol-1-yl)methyl)-1H-1,2,3-triazole; potassium acetate In dichloromethane for 24h; Inert atmosphere; Reflux; optical yield given as %ee; enantioselective reaction; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 35℃; for 1h; Solvent; Time; | 99% |
Diallylamine Consensus Reports
Diallylamine Standards and Recommendations
Diallylamine Specification
1. Introduction of Diallylamine
Diallylamine has the IUPAC name of N-prop-2-enylprop-2-en-1-amine. It is a kind of clear colourless to yellow liquid and belongs to the classes of Pharmaceutical Intermediates; Acyclic; Alkenes; Organic Building Blocks. And it should be stored in a cool, ventilated and dry place.
2. Properties of Diallylamine
Physical properties of Diallylamine are: (1)ACD/LogP: 1.11 ; (2)#H bond acceptors: 1; (3)#H bond donors: 1; (4)#Freely Rotating Bonds: 4; (5)Polar Surface Area: 12.03 Å2; (6)Index of Refraction: 1.432; (7)Molar Refractivity: 32.89 cm3; (8)Molar Volume: 126.5 cm3; (9)Polarizability: 13.03 ×10-24cm3; (10)Surface Tension: 22.8 dyne/cm; (11)Density: 0.767 g/cm3; (12)Flash Point: 15.6 °C; (13)Enthalpy of Vaporization: 35.37 kJ/mol; (14)Boiling Point: 115.3 °C at 760 mmHg; (15)Vapour Pressure: 19.2 mmHg at 25°C.
3. Structure Descriptors of Diallylamine
You could convert the following datas into the molecular structure:
(1)SMILES: C=CCNCC=C
(2)InChI: InChI=1/C6H11N/c1-3-5-7-6-4-2/h3-4,7H,1-2,5-6H2
(3)InChIKey: DYUWTXWIYMHBQS-UHFFFAOYAO
4. Toxicity of Diallylamine
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LC50 | inhalation | 2100mg/m3 (2100mg/m3) | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 14, Pg. 80, 1975. | |
| mammal (species unspecified) | LD50 | unreported | 350mg/kg (350mg/kg) | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 14, Pg. 80, 1975. | |
| man | TCLo | inhalation | 5ppm/5M (5ppm) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE LUNGS, THORAX, OR RESPIRATION: STRUCTURAL OR FUNCTIONAL CHANGE IN TRACHEA OR BRONCHI | Archives of Environmental Health. Vol. 1, Pg. 343, 1960. |
| mouse | LD50 | intraperitoneal | 187mg/kg (187mg/kg) | BEHAVIORAL: TREMOR GASTROINTESTINAL: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Archives of Environmental Health. Vol. 1, Pg. 343, 1960. |
| mouse | LD50 | oral | 355mg/kg (355mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 43, 1982. | |
| rabbit | LD50 | skin | 280uL/kg (0.28mL/kg) | American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. | |
| rat | LC50 | inhalation | 795ppm/8H (795ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Archives of Environmental Health. Vol. 1, Pg. 343, 1960. |
| rat | LD50 | oral | 578mg/kg (578mg/kg) | BEHAVIORAL: TREMOR LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: OTHER CHANGES | Archives of Environmental Health. Vol. 1, Pg. 343, 1960. |
5. Preparation of Diallylamine
Preparation of Diallylamine: it can be prepared by diallylcyanoamine through hydrolysis in the presence of sulfuric acid. This reaction should reflux for 6 hours, and the yield is about 80-88%. In addition, it also can be prepared by diallyl-carbamic acid 1-(3,5-dimethoxy-phenyl)-2-oxo-2-phenyl-ethyl ester. The other product is 5,7-dimethoxy-2-phenyl-benzofuran. This reaction will need reagent benzene with irradiation. The yield is about 56%. The equation as follows:
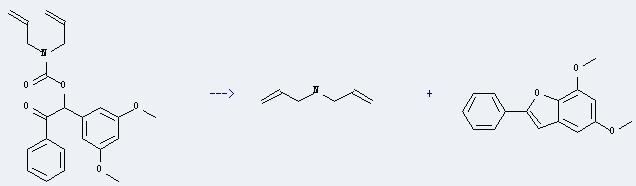
6. Uses of Diallylamine
Uses of Diallylamine: this chemical can be used as intermediates in organic synthesis. And it can react with acryloyl chloride to get diallylacrylamide. This reaction will need reagent Et3N and solvent benzene. The reaction time is 30 minutes at reaction temperature of 0 °C. And the yield is about 99%.
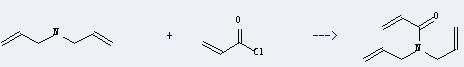
7. Safety information of Diallylamine
Hazard Codes: F,T
Risk Statements: 11-20/22-24-34
Safety Statements: 16-26-36/37/39-45-28A
RIDADR UN: 2359 3/PG 2
WGK Germany: 3
RTECS: UC6650000
F: 2-10
HazardClass: 3
PackingGroup: II
Hazardous Substances Data: 124-02-7(Hazardous Substances Data)
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable and can cause burns. In addition, it is toxic in contact with skin and harmful by inhalation and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show label where possible). What's more, you should keep away from sources of ignition.
Related Products
- Diallylamine
- Diallylamine hydrochloride
- 124027-47-0
- 124035-23-0
- 124-03-8
- 124038-37-5
- 1240390-36-6
- 124041-00-5
- 124044-11-7
- 124044-13-9
- 124044-21-9
- 124044-66-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View