-
Name
Dicyclohexylamine
- EINECS 202-980-7
- CAS No. 101-83-7
- Article Data258
- CAS DataBase
- Density 0.912 g/cm3
- Solubility 1 g/L (20 °C) in water
- Melting Point -2 °C
- Formula C12H23N
- Boiling Point 256.1 °C at 760 mmHg
- Molecular Weight 181.321
- Flash Point 96.1 °C
- Transport Information UN 2565 8/PG 3
- Appearance Colorless liquid
- Safety 26-36/37/39-45-60-61
- Risk Codes 22-34-50/53
-
Molecular Structure
-
Hazard Symbols
 C,
C, N
N
- Synonyms Dodecahydrodiphenylamine;N,N-Dicyclohexylamine;N-Cyclohexylcyclohexanamine;NSC 3399;Dicyclohexylamine(8CI);Aminodicyclohexane;Bis(cyclohexyl)amine;D-CHA-T;
- PSA 12.03000
- LogP 3.63230
Synthetic route
-
-
62-53-3
aniline

-
A
-
110-82-7
cyclohexane

-
B
-
108-91-8
cyclohexylamine

-
C
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
D
-
110-83-8
cyclohexene

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen at 180 - 200℃; | A n/a B 98.4% C 0.08% D n/a |
| With hydrogen at 160 - 200℃; under 150015 Torr; | A n/a B 95.9% C 0.45% D n/a |


| Conditions | Yield |
|---|---|
| With lithium hydroxide; hydrogen; 5% ruthenium/lithium aluminate In water at 150℃; under 44718.8 Torr; for 2.08333h; Product distribution / selectivity; Neat (no solvent); | A 97.1% B 1% |
| With lithium hydroxide; hydrogen; 5% activated charcoal-supported ruthenium catalyst In water at 150℃; under 44718.8 Torr; for 1.21667h; Product distribution / selectivity; Neat (no solvent); | A 91.8% B 5.73% |
| With hydrogen; 5% ruthenium/lithium aluminate at 150℃; under 44718.8 Torr; for 1.28333 - 3h; Product distribution / selectivity; Neat (no solvent); | A 89.2% B 0.08% |

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| Stage #1: dicyclohexyl-(3-methylbut-2-enyl)amine With 2,2'-azobis(isobutyronitrile); para-thiocresol In benzene Heating; Stage #2: With hydrogenchloride | 97% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 20℃; for 4h; chemoselective reaction; | 95% |
-
-
39830-56-3
N,N-dicyclohexyl-p-toluenesulfonamide

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With pyrrolidine; samarium diiodide; water In tetrahydrofuran at 20℃; Inert atmosphere; | 96% |
| With naphthalene; tetraethylammonium bromide In N,N-dimethyl-formamide at 0℃; Inert atmosphere; Electrolysis; | 96% |
| Stage #1: N,N-dicyclohexyl-p-toluenesulfonamide With Na/K absorbed into silica gel In tetrahydrofuran at 20℃; Inert atmosphere; Stage #2: With water In tetrahydrofuran | 83% |
| With diammonium hydrogenphosphate In tetrahydrofuran at 20℃; Product distribution / selectivity; Cooling with ice; | 80% |

| Conditions | Yield |
|---|---|
| With {[(PCy3)(CO)RuH]4(μ-O)(μ-OH)2}; 4-tert-Butylcatechol In chlorobenzene at 130℃; for 16h; Glovebox; Schlenk technique; Sealed tube; chemoselective reaction; | 95% |
| With tris(triphenylphosphine)ruthenium(II) chloride In tetrahydrofuran at 185℃; for 5h; | 92% |
| With NiCuFeO(x) In 5,5-dimethyl-1,3-cyclohexadiene for 24h; Inert atmosphere; Sealed tube; Reflux; | 82% |

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| Stage #1: ((E)-But-2-enyl)-dicyclohexyl-amine With 2,2'-azobis(isobutyronitrile); para-thiocresol In benzene Heating; Stage #2: With hydrogenchloride | 95% |

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With formic acid; potassium hydroxide In ethanol at 70℃; for 1h; | 93% |
| With Pd(OH)2/C In methanol for 8h; Reflux; | 62% |

| Conditions | Yield |
|---|---|
| With NiCuFeO(x) In 5,5-dimethyl-1,3-cyclohexadiene for 24h; Inert atmosphere; Sealed tube; Reflux; | 92% |
| With lithium hydroxide In neat (no solvent) at 140℃; for 48h; Inert atmosphere; | 74% |
| With nickel at 180 - 190℃; im Ruehrautoklaven; | |
| With thorium dioxide at 290 - 320℃; |

| Conditions | Yield |
|---|---|
| With urea In 1,3,5-trimethyl-benzene at 141℃; under 760.051 Torr; for 16h; Inert atmosphere; | 92% |
| With ammonium tetrafluroborate; sodium hydrogencarbonate; bis[dichloro(pentamethylcyclopentadienyl)iridium(III)] at 140℃; for 17h; | 86% |
| With [Cp*Ir(NH3)3]I2; ammonia In water at 140℃; for 24h; Autoclave; Inert atmosphere; | 85% |

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; methoxybenzene In dichloromethane at 0℃; desulfonation; | 92% |
-
-
10468-40-3
cyclohexanone N-cyclohexylimine

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate; bis(η5-cyclopentadinyl)dihydridomolybdenum In methanol at 50℃; for 24h; | 90% |
| With ytterbium(III) triflate; bis(η5-cyclopentadinyl)dihydridomolybdenum In methanol at 50℃; for 24h; Product distribution; other reag.; other solvent; other temp.; other reaction time; varying amount of catalyst; | 90% |
| With lithium; nickel dichloride In tetrahydrofuran for 6h; Ambient temperature; | 78% |
-
-
162466-77-5
N-allyl-N-cyclohexylcyclohexanamine

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 20℃; for 18h; chemoselective reaction; | 90% |
| With Pd(OH)2/C In methanol for 1h; Reflux; | 69% |
| Stage #1: N-allyl-N-cyclohexylcyclohexanamine With 2,2'-azobis(isobutyronitrile); para-thiocresol In benzene Heating; Stage #2: With hydrogenchloride | 63% |
| With titanium(III) chloride; lithium In tetrahydrofuran for 20h; Heating; | 47% |
| With water; [Ru(η3:η2:η3-dodeca-2,6,10-triene-1,12-diyl)Cl2] at 90℃; for 1.2h; | 99 % Chromat. |

| Conditions | Yield |
|---|---|
| With ammonium acetate In benzene for 1.5h; Leuckart type reaction; Reflux; | 85% |
| With N,N,N'N'-tetramethyl-1,3-propanediamine; carbon monoxide; water; hexarhodium hexadecacarbonyl In 2-ethoxy-ethanol at 80℃; under 6080 Torr; for 5h; Mechanism; analogous reaction of other ketones; | 80% |
| With N,N,N'N'-tetramethyl-1,3-propanediamine; carbon monoxide; water; hexarhodium hexadecacarbonyl In 2-ethoxy-ethanol at 80℃; under 6080 Torr; for 5h; Mechanism; analogous reaction with other ketones, analogous reaction of an other amine; | 80% |

| Conditions | Yield |
|---|---|
| With dichloro(μ-chloro)(μ-hydrido)bis(η-p-cymene)diruthenium(II); hydrogen In 1,4-dioxane at 90℃; under 37503.8 Torr; for 40h; | 82% |
| With nickel at 150℃; under 91938.4 - 147102 Torr; Hydrogenation.ohne Loesungsmittel; | |
| With nickel; methyl cyclohexane at 175℃; under 147102 - 161812 Torr; Hydrogenation; |
-
-
108-91-8
cyclohexylamine

-
-
108-88-3
toluene

-
A
-
1821-36-9
N-phenyl-2-cyclohexylamine

-
B
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With Co2Rh2/C at 180℃; under 760.051 Torr; for 18h; Temperature; Autoclave; Inert atmosphere; | A 12% B 82% |

-
-
62-53-3
aniline

-
A
-
108-94-1
cyclohexanone

-
B
-
108-91-8
cyclohexylamine

-
C
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
D
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen; silica gel; rhodium In water at 49.9℃; under 15151.2 Torr; Product distribution; Other catalysts, other temperatures.; | A n/a B 81.5% C 2.8% D 15.7% |
| With hydrogen; 5 percent Rh/MgO; magnesium oxide In water at 49.9℃; under 15001.2 Torr; Product distribution; Hydrogenation in the presence of cyclohexylamine; cyclohexanone; cyclohexanol; dicyclohexylamine; ammonia and mixture of them.; | A 0.8% B 73.2% C 6.6% D 18.8% |
| With hydrogen; Ru(OH)Cl3 In water at 99.9℃; under 30002.4 Torr; Kinetics; Rate constant; Product distribution; energy data; variation of temperature, pressure; also in presence of oxides; activation energy; | A 1.5% B 64.7% C 1% D 32.5% |

-
-
62-53-3
aniline

-
A
-
1821-36-9
N-phenyl-2-cyclohexylamine

-
B
-
108-91-8
cyclohexylamine

-
C
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| aluminum oxide; ruthenium at 170℃; for 2.5h; Product distribution; Mechanism; investigation of the hydrogenation of aniline with various hydrogenation catalysts in the presence and abscence of the fused salts; | A 0.3% B 80.4% C 7% |
| With hydrogen; palladium/alumina at 240℃; for 2.5h; Product distribution; Mechanism; investigation of the hydrogenation of aniline with various hydrogenation catalysts in the presence and abscence of the fused salts; | A 1.1% B 20% C 51.9% |
| With formic acid; palladium on activated charcoal In methanol for 70h; Ambient temperature; | A 49 % Chromat. B 20 % Chromat. C 31 % Chromat. |

-
-
19520-87-7
2-Dicyclohexylamino-ethylacetat

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With bis(p-methoxyphenyl)methanone; water In acetonitrile for 3h; deprotection; Irradiation; | 80% |
-
-
108-94-1
cyclohexanone

-
-
62-53-3
aniline

-
A
-
108-91-8
cyclohexylamine

-
B
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexanone; aniline With hydrogen at 120℃; under 15001.5 Torr; Stage #2: at 180℃; Pressure; Temperature; | A 20.2% B 78.3% |
| Stage #1: cyclohexanone; aniline With ammonia; hydrogen at 150℃; under 37503.8 Torr; Stage #2: at 170℃; Reagent/catalyst; Temperature; Pressure; | A 65.3% B 32.8% |

-
-
82259-52-7
1,1-dicyclohexylhydrazine

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With titanium(III) chloride; water In ethanol pH=7; Reflux; Alkaline aq. solution; Inert atmosphere; | 76% |
| With titanium tetrachloride; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; | 70% |
-
-
100504-97-0
N,N-dicyclohexylmethanesulfonamide

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dicyclohexylmethanesulfonamide With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.25h; Inert atmosphere; Stage #2: With oxygen In tetrahydrofuran; hexane at 20℃; for 1h; Stage #3: With water In tetrahydrofuran; hexane | 75% |
-
-
108-91-8
cyclohexylamine

-
-
108-93-0
cyclohexanol

-
A
-
10468-40-3
cyclohexanone N-cyclohexylimine

-
B
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With Mg-Al hydrotalcite supported copper at 180℃; for 15h; | A 74% B 26% |
| With C27H19ClNO3PRu; potassium tert-butylate at 110 - 150℃; for 4h; Inert atmosphere; | A 38 %Spectr. B 9 %Spectr. |

-
-
1195-42-2
N-cyclohexylisopropylamine

-
A
-
108-91-8
cyclohexylamine

-
B
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With dicarbonyl(chloro)(η5-pentaphenylcyclopentadienyl)ruthenium(II); ammonia In tert-Amyl alcohol at 170℃; for 23.5h; Inert atmosphere; Schlenk technique; Autoclave; | A 74% B 7.5% |

| Conditions | Yield |
|---|---|
| With Rh/Al2O3; hydrogen In tetrahydrofuran; water at 80℃; under 15001.5 Torr; for 24h; | A 73% B 27% |
| With 5 wt% ruthenium/carbon; hydrogen; sodium nitrite In tetrahydrofuran at 170℃; under 62256.2 Torr; for 4h; Solvent; Autoclave; | |
| With dodecane; 5 wt% ruthenium/carbon; hydrogen In tetrahydrofuran at 140℃; under 75007.5 Torr; for 3h; Reagent/catalyst; Autoclave; | |
| With hydrogen; sodium chloride In isopropyl alcohol at 90℃; under 45004.5 Torr; for 0.833333h; Catalytic behavior; Reagent/catalyst; |

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| Stage #1: (E)-N,N-dicyclohexyl-3-phenyl-2-propenylamine With 2,2'-azobis(isobutyronitrile); para-thiocresol In benzene Heating; Stage #2: With hydrogenchloride | 70% |
-
-
98-95-3
nitrobenzene

-
A
-
108-91-8
cyclohexylamine

-
B
-
62-53-3
aniline

-
C
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With octylated silica; hydrogen; sodium 4-dodecylbenzenesulfonate; {[(CH3)(C8H17)3N](+)[RhCl4](-)} at 80℃; under 10343 Torr; for 24h; | A 25% B 6% C 69% |
| With propane; hydrogen at 200 - 250℃; under 60006 Torr; Supercritical conditions; Flow reactor; | A 29 %Chromat. B 9 %Chromat. C 58 %Chromat. |
| With dodecane; 5 % platinum on carbon; hydrogen In tetrahydrofuran at 140℃; under 75007.5 Torr; for 3h; Reagent/catalyst; Autoclave; |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; tert-butylimino-tri(pyrrolidino)phosphorane; 1,1'-bi-2-naphthol In N,N-dimethyl-formamide; acetonitrile at -10℃; for 24h; Irradiation; | 69% |
-
-
10468-40-3
cyclohexanone N-cyclohexylimine

-
A
-
108-91-8
cyclohexylamine

-
B
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel boride In methanol Yield given; | A 68% B n/a |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel boride In methanol Yields of byproduct given; | A 68% B n/a |
| With Pt/Al2O3; hydrogen In ethanol at 100℃; under 4500.45 Torr; for 3h; Autoclave; chemoselective reaction; |
-
-
110-89-4
piperidine

-
-
108-91-8
cyclohexylamine

-
A
-
3319-01-5
1-cyclohexylpiperidine

-
B
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With platinum-nickel nanoclusters on activated carbon; hydrogen at 160℃; under 760.051 Torr; Flow reactor; chemoselective reaction; | A 62% B 26.3% |


| Conditions | Yield |
|---|---|
| Stage #1: N-cyclohexyl-cyclohexanamine With n-butyllithium In tetrahydrofuran Metallation; Stage #2: With nitrogen(II) oxide In tetrahydrofuran at -78℃; for 3h; Nitrosation; | 100% |
| Stage #1: N-cyclohexyl-cyclohexanamine With n-butyllithium In hexane Stage #2: With nitric oxide In tetrahydrofuran under 759811 Torr; for 3h; cooling; | 100% |
| With [NO(1+)*18-crown-6*H(NO3)2(1-)] In dichloromethane at 20℃; for 0.0833333h; | 100% |
-
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
-
106-89-8
epichlorohydrin

-
-
150389-77-8
1-Chloro-3-dicyclohexylamino-propan-2-ol

| Conditions | Yield |
|---|---|
| In ethanol for 24h; Heating; | 100% |
-
-
31562-43-3
tert-butylsulfinyl chloride

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; sulfinylation; | 100% |

| Conditions | Yield |
|---|---|
| chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium (II) at 100℃; | 100% |
| With TiO2 supported nano-Pd(0.8) catalyst In water at 20℃; for 15h; Inert atmosphere; Irradiation; Green chemistry; | 93% |
| With [Cp*Ir(2-(1H-benzo[d]imidazol-2-yl)-1H-benzo[d]imidazole)Cl][Cl]; caesium carbonate at 120℃; for 12h; Schlenk technique; | 82% |
-
-
50-00-0
formaldehyd

-
-
536-74-3
phenylacetylene

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
-
801304-96-1
N-cyclohexyl-N-(3-phenylprop-2-yn-1-yl)cyclohexanamine

| Conditions | Yield |
|---|---|
| With copper(l) iodide In water at 25℃; for 1.5h; Mannich reaction; | 100% |
| With polystyrene-supported 1-benzyl-1H-imidazole-Ag(I) catalyst at 20℃; for 24h; Inert atmosphere; | 97% |
| copper(l) iodide In water; dimethyl sulfoxide at 30℃; for 20h; | 96% |

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With perchloric acid at 30 - 35℃; for 0.75h; | 100% |
| at 20℃; for 0.166667h; Ionic liquid; | 100% |
| With 1,4-disulfopiperazine-1,4-diium chloride In neat (no solvent) at 20℃; for 1h; Green chemistry; chemoselective reaction; | 96% |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
-
935753-45-0
2,2-dicyclohexyl-1-butylidenehydrazine

| Conditions | Yield |
|---|---|
| Stage #1: N-cyclohexyl-cyclohexanamine With n-butyllithium In hexane Stage #2: With nitric oxide In tetrahydrofuran under 759811 Torr; for 3h; cooling; Stage #3: n-butyllithium In tetrahydrofuran; hexane | 100% |
-
-
135077-83-7
3-(1-adamantyl)-4-vinylbenzoic acid

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
-
135077-84-8
3-(1-adamantyl)-4-vinylbenzoyl chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride In diethyl ether; dichloromethane | 100% |
-
-
120-80-9
benzene-1,2-diol

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
-
76128-65-9
N-(trimethoxysilylmethyl)hexahydroazepin-2-one

| Conditions | Yield |
|---|---|
| In o-xylene at 130 - 140℃; | 100% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether; water at 20℃; for 0.5h; | 100% |
| With hydrogenchloride In 1,4-dioxane; water at 20℃; for 12h; | 98% |
| With hydrogenchloride In diethyl ether; hexane Inert atmosphere; |
-
-
18934-81-1
N-(tert-butyloxycarbonyl)-12-aminododecanoic acid

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
-
943923-29-3
dicyclohexylammonium 12-((tert-butoxycarbonyl)amino)dodecanoate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.166667h; | 100% |
| In methanol at 20℃; for 0.166667h; |
-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| With hydrogen iodide In 1,4-dioxane; water at 20℃; for 12h; | 100% |
| With hydrogen iodide In diethyl ether; water at 20℃; for 0.5h; | 79% |
-
-
101-83-7
N-cyclohexyl-cyclohexanamine

-
-
73254-21-4
dicyclohexylamine hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In 1,4-dioxane; water at 20℃; for 12h; | 100% |
| With hydrogen bromide In diethyl ether; water at 20℃; for 0.5h; | 74% |

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; Inert atmosphere; | 100% |
| With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; for 24h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: N-cyclohexyl-cyclohexanamine With n-butyllithium In diethyl ether; hexane at -78 - 0℃; for 0.5h; Inert atmosphere; Stage #2: N,N-dimethylaminodichlorophosphane In diethyl ether; cyclohexane at 20℃; for 0.75h; Inert atmosphere; | 100% |

-
-
101-83-7
N-cyclohexyl-cyclohexanamine

| Conditions | Yield |
|---|---|
| In water at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |
Dicyclohexylamine Consensus Reports
Dicyclohexylamine Standards and Recommendations
Dicyclohexylamine Specification
The N,N-Dicyclohexylamine, with the CAS registry number 101-83-7, is also known as Dodecahydrodiphenylamine. It belongs to the product categories of Pharmaceutical Intermediates; Amines; C11 to C38; Nitrogen Compounds; Aliphatics Volatiles/ Semivolatiles; Alpha Sort; Chemical Class; D; DAlphabetic; DIA - DIC; Analytical Reagents for General Use; C-D, Puriss p.a.; Puriss p.a.. Its EINECS registry number is 202-980-7. This chemical's molecular formula is C12H23N and molecular weight is 181.31772. Its IUPAC name is called N-cyclohexylcyclohexanamine. This chemical's classification codes are Enzyme inhibitors; Mutation Data; Skin / Eye Irritant; Tumor Data.
Physical properties of N,N-Dicyclohexylamine: (1)ACD/LogP: 3.69; (2)ACD/LogD (pH 5.5): 0.59; (3)ACD/LogD (pH 7.4): 0.64; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1.92; (7)ACD/KOC (pH 7.4): 2.15; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 2; (11)Index of Refraction: 1.487; (12)Molar Refractivity: 57.12 cm3; (13)Molar Volume: 198.2 cm3; (14)Surface Tension: 33.1 dyne/cm; (15)Density: 0.91 g/cm3; (16)Flash Point: 96.1 °C; (17)Enthalpy of Vaporization: 49.36 kJ/mol; (18)Boiling Point: 256.1 °C at 760 mmHg; (19)Vapour Pressure: 0.0157 mmHg at 25°C.
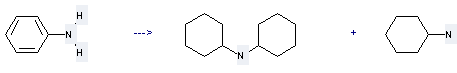
Preparation of N,N-Dicyclohexylamine: this chemical can be prepared by aniline. This reaction will need reagent nickel catalyst.
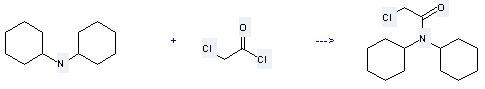
Uses of N,N-Dicyclohexylamine: it can be used to produce chloro-acetic acid dicyclohexylamide at temperature of 25 °C. This reaction will need reagent CH2Cl2 with reaction time of 12 hours. The yield is about 61%.
When you are using this chemical, please be cautious about it as the following:
This chemical may destroy living tissue on contact and may cause burns. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1CCC(CC1)NC2CCCCC2
(2)InChI: InChI=1S/C12H23N/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h11-13H,1-10H2
(3)InChIKey: XBPCUCUWBYBCDP-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 500mg/kg (500mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 468, 1986. | |
| mouse | LD50 | subcutaneous | 135mg/kg (135mg/kg) | Voprosy Onkologii. Problems of Oncology. For English translation, see PONCAU. Vol. 4, Pg. 659, 1958. | |
| rabbit | LDLo | subcutaneous | 500mg/kg (500mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Industrial and Engineering Chemistry. Vol. 29, Pg. 1247, 1937. |
| rat | LD50 | oral | 373mg/kg (373mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 468, 1986. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View