-
Name
Diethyl allylmalonate
- EINECS 218-072-9
- CAS No. 2049-80-1
- Article Data145
- CAS DataBase
- Density 1.021 g/cm3
- Solubility Not miscible or difficult to mix with water.
- Melting Point
- Formula C10H16O4
- Boiling Point 222.499 °C at 760 mmHg
- Molecular Weight 200.235
- Flash Point 92.778 °C
- Transport Information
- Appearance clear colorless to slightly yellow liquid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Malonicacid, allyl-, diethyl ester (6CI,7CI,8CI);Propanedioic acid, 2-propenyl-,diethyl ester (9CI);(2-Propenyl)propanedioic acid diethyl ester;2-Allylmalonic acid diethyl ester;Allylmalonic acid diethyl ester;Diethyl1-butene-4,4-dicarboxylate;Diethyl 2-allyl-1,3-propanedioate;Diethyl2-allylmalonate;Diethyl 2-prop-2-enylmalonate;Diethyl 2-propenylmalonate;Ethyl allylmalonate;NSC 67393;NSC 8777;
- PSA 52.60000
- LogP 1.30490
Synthetic route
-
-
106-95-6
allyl bromide

-
-
105-53-3
diethyl malonate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With tetrabutyl-ammonium chloride; potassium carbonate In dichloromethane; water at 80℃; for 45h; | 100% |
| 98% | |
| With P(MeNCH2CH2)3N In hexane; acetonitrile at 25℃; for 0.5h; | 94% |
-
-
591-87-7
Allyl acetate

-
-
105-53-3
diethyl malonate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium fluoride on basic alumina for 0.0833333h; Trost-Tsuji reaction; microwave irradiation; | 98% |
| With C29H29N2O2PPd; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 15h; Reagent/catalyst; Tsuji-Trost Allylation; Schlenk technique; Inert atmosphere; | 77% |
| With potassium fluoride on basic alumina; 1,2-bis-(diphenylphosphino)ethane; bis[1,2-bis(diphenylphosphino)ethane]palladium(0) In tetrahydrofuran for 4.5h; Ambient temperature; | 75% |
-
-
107-18-6
allyl alcohol

-
-
105-53-3
diethyl malonate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With pyridine; magnesium sulfate; 1,2-(p-MeOC6H4)2cyclobuten[=P(2,4,6-tri-t-BuPh)]2PdOTf at 50℃; for 12h; | 92% |
| With [bis(methyldiphenylsilyl)methyl]diphenylphosphine; pyridine; tetrabutyl ammonium fluoride; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane at 100℃; for 8h; Tsuji-Trost reaction; | 86% |
-
-
591-87-7
Allyl acetate

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With C33H33N2O4PPd; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 15h; Reagent/catalyst; Tsuji-Trost Allylation; Schlenk technique; Inert atmosphere; | A 91% B 8% |
| With C48H47ClN2P2Pd(1+); potassium carbonate In N,N-dimethyl-formamide at 20℃; for 15h; Reagent/catalyst; Tsuji-Trost Allylation; Schlenk technique; Inert atmosphere; | A 22% B 78% |
| With aluminum oxide; triphenylphosphine; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 40h; Ambient temperature; | A 70% B 15% |

-
-
107-05-1
3-chloroprop-1-ene

-
-
105-53-3
diethyl malonate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate In acetonitrile at 50℃; for 7h; | 87% |
| With N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate In acetonitrile for 7h; | 87% |
-
-
74070-96-5
Phenyl-phosphonothioic acid S-allyl ester O-ethyl ester

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate; tetrakis(triphenylphosphine) palladium(0) at 80℃; for 0.666667h; Product distribution; | A 87% B 13% |

-
-
16515-85-8
triethyl pent-4-ene-1,2,2-tricarboxylate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium ethanolate In tetrahydrofuran for 1h; Inert atmosphere; | 87% |
-
-
35466-83-2
allyl methyl carbonate

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With triphenylphosphine In water at 50℃; for 24h; | A 84% B 4% |
| With triphenylphosphine; Pd-Gr In tetrahydrofuran; dodecane for 4h; Heating; Title compound not separated from byproducts; | A 41 % Chromat. B 3 % Chromat. |
| With tris-(dibenzylideneacetone)dipalladium(0); 2,2’-(methylenedisulfanediyl)bis(1,3-benzothiazole) In tetrahydrofuran at 70℃; for 2h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Tsuji-Trost Allylation; chemoselective reaction; |


| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 20℃; for 16h; | 82% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran | 80% |
-
-
34727-00-9
lithium diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide; diethyl ether for 1.5h; -78 deg C -> r.t.; Title compound not separated from byproducts; | A 13% B 76% |

-
-
106-95-6
allyl bromide

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: diethyl malonate With sodium hydride In tetrahydrofuran at 0℃; for 0.25h; Stage #2: allyl bromide In tetrahydrofuran at 0 - 25℃; for 14h; Further stages.; | A 24% B 76% |
| Stage #1: diethyl malonate With sodium hydride In tetrahydrofuran at 0 - 20℃; for 1h; Stage #2: allyl bromide at 20℃; for 12h; Inert atmosphere; | A 68% B n/a |
| Stage #1: diethyl malonate With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: allyl bromide In tetrahydrofuran at 20℃; for 12h; | A 68% B 12% |

-
-
17686-63-4
acetone O-<(allyloxy)-carbonyl>oxime

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| ethyl-diphenyl-phosphane; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran for 1h; Ambient temperature; | A 75% B 3% |

-
-
118068-86-3
C12H13NO3

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| ethyl-diphenyl-phosphane; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran for 1.5h; Product distribution; Ambient temperature; variation of allylating agents, solvent, temperature and reaction time.; | A 75% B 14% |
| ethyl-diphenyl-phosphane; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran for 1.5h; Ambient temperature; | A 75% B 14% |

-
-
1823-91-2
1-phenylethyl cyanide

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate; Pd(PhCH=CH)2CO>2CO; 1,2-bis-(diphenylphosphino)ethane In N,N-dimethyl-formamide Ambient temperature; | A 74% B 26% |

-
-
105-53-3
diethyl malonate

-
-
762-72-1
allyl-trimethyl-silane

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In methanol for 4h; Ambient temperature; | 74% |
| With ammonium cerium (IV) nitrate In methanol | 57% |
-
-
74070-90-9, 97714-35-7, 97714-36-8
Allyl ethyl phenylphosphonothionate

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

-
C
-
74070-96-5
Phenyl-phosphonothioic acid S-allyl ester O-ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate; tetrakis(triphenylphosphine) palladium(0) at 80℃; for 0.333333h; Product distribution; | A 25% B 2% C 73% |

-
-
556-56-9
allyl iodid

-
-
105-53-3
diethyl malonate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With cerium oxide nanoparticle In water at 20℃; for 12h; Green chemistry; | 72% |
| Stage #1: diethyl malonate With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 1h; Stage #2: allyl iodid In tetrahydrofuran for 24h; Reflux; |
-
-
107-05-1
3-chloroprop-1-ene

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: diethyl malonate With potassium tert-butylate In N,N-dimethyl-formamide at 0℃; for 0.0833333h; Stage #2: 3-chloroprop-1-ene In N,N-dimethyl-formamide at 20℃; for 12h; | A 70% B 5% |
| With potassium hydroxide; polyethylene glycol Alkylation; | A 42.5% B n/a |
| With potassium hydroxide; water; cetyltrimethylammonim bromide for 1h; Alkylation; | A 30.9% B n/a |
| With ethanol; sodium |

-
-
24850-33-7
allyltributylstanane

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With [Cu(2,9-bis(4-methoxyphenyl)-1,10-phenanthroline)2]Cl In acetonitrile for 4.5h; Irradiation; Inert atmosphere; | 70% |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
762-72-1
allyl-trimethyl-silane

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With fac-tris[2-phenylpyridinato-C2,N]iridium(III) In acetonitrile at 20 - 30℃; for 24h; Inert atmosphere; Irradiation; | 70% |
| With C44H29CuN4O3P(1+)*BF4(1-) In acetonitrile at 20℃; Reagent/catalyst; UV-irradiation; | 64% |
-
-
118068-88-5
C10H15NO3

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| ethyl-diphenyl-phosphane; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran for 3h; Ambient temperature; | A 69% B 2% |

-
-
50998-68-0
4-nitro-benzaldehyde-(O-allyl-seqtrans-oxime )

-
-
105-53-3
diethyl malonate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium diethylmalonate; ethyl-diphenyl-phosphane; bis(dibenzylideneacetone)-palladium(0) In N,N-dimethyl-formamide at 100℃; for 24h; | 67% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; eosin y In methanol at 20℃; Irradiation; | 67% |
-
-
996-82-7, 34727-00-9, 73177-21-6
sodium diethylmalonate

-
-
29443-23-0
N,N,N-triethylprop-2-en-1-aminium bromide

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 24h; Ambient temperature; | A 62% B 13% |

-
-
996-82-7, 34727-00-9, 73177-21-6
sodium diethylmalonate

-
-
591-87-7
Allyl acetate

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| diiron nonacarbonyl In tetrahydrofuran at 20℃; for 120h; | 61% |
-
-
107-18-6
allyl alcohol

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With acetic acid; tetrakis(triphenylphosphine) palladium(0) at 100℃; for 0.166667h; | A 61% B 30% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; nickel(II) bromide trihydrate; tetrabutylammonium acetate; zinc In N,N-dimethyl acetamide at 50℃; for 66h; Inert atmosphere; | A 45% B 6% |
| With bis(1,5-cyclooctadiene)nickel (0); 1,4-di(diphenylphosphino)-butane In toluene at 105℃; for 17h; Reagent/catalyst; Temperature; Inert atmosphere; Schlenk technique; | A 20 %Chromat. B 63 %Chromat. |


| Conditions | Yield |
|---|---|
| With iodine In dimethyl sulfoxide at 60℃; for 0.5h; Acidic conditions; | 57% |
| With tris(triphenylphosphine)ruthenium(II) chloride; triethylaluminum In toluene at 20℃; for 36h; | 91 % Spectr. |
| With triethylaluminum; Fe(III)bis(imidazolonyl)pyridine)Cl3 In toluene at 20℃; for 24h; | 13 % Spectr. |
-
-
85021-16-5
allyl (N-phenyl)benzimidate

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With 1,2-bis-(diphenylphosphino)ethane; bis(dibenzylideneacetone)-palladium(0) In N,N-dimethyl-formamide for 4h; Ambient temperature; | A 55% B 28% |

-
-
107-11-9
1-amino-2-propene

-
-
105-53-3
diethyl malonate

-
A
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
B
-
3195-24-2
diallylmalonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With nickel(II) bromide trihydrate; 1,4-di(diphenylphosphino)-butane; zinc In N,N-dimethyl-formamide at 80℃; for 22h; Inert atmosphere; | A 55% B 20% |

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
13353-23-6
ethyl (±)-4,5-epoxy-2-(ethoxycarbonyl)pentanoate

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane for 15h; Ambient temperature; | 100% |
| With 3-chloro-benzenecarboperoxoic acid In chloroform for 12h; Ambient temperature; | 98% |
| With potassium peroxymonosulfate sulfate In aq. phosphate buffer; water; ethyl acetate; acetone at 20℃; pH=8; | 86% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol | 100% |
| With sodium hydroxide In methanol; dichloromethane at 20℃; for 1h; | 87% |
| With lithium aluminium tetrahydride In tetrahydrofuran | 85% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
1528-30-9
methylenecyclopentane

-
-
127220-05-7
diethyl 4-methylenespiro<4.4>nonane-2,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With copper diacetate; manganese triacetate In acetic acid at 70 - 75℃; for 24h; | 100% |
-
-
5332-26-3
N-(bromomethyl)phtalimide

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
1200242-48-3
diethyl 2‐allyl‐2‐phthalimidomethylmalonate

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 0 - 25℃; Inert atmosphere; Stage #2: N-(bromomethyl)phtalimide In tetrahydrofuran at 0 - 25℃; Inert atmosphere; | 100% |
-
-
50-00-0
formaldehyd

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
82515-47-7
diethyl 2-allyl-2-(hydroxymethyl)malonate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol; water at 0 - 20℃; for 20h; | 99% |
| With sodium hydrogencarbonate In ethanol; water at 0 - 20℃; for 20h; | 99% |
| With potassium carbonate In dimethyl sulfoxide for 168h; Ambient temperature; | 13% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
18495-26-6
1-bromo-2-heptyne

-
-
191801-57-7
diethyl 2-allyl-2-(hept-2-yn-1-yl)malonate

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 1h; Inert atmosphere; Stage #2: 1-bromo-2-heptyne In tetrahydrofuran; mineral oil at 0 - 20℃; for 12h; Inert atmosphere; | 99% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 20℃; Stage #2: 1-bromo-2-heptyne In tetrahydrofuran at 20℃; for 12h; Further stages.; |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
161678-48-4
Octa-<(3-mercaptopropyl)-silsesquioxan>

| Conditions | Yield |
|---|---|
| With 2,2-dimethoxy-2-phenylacetophenone In tetrahydrofuran for 0.5h; UV-irradiation; | 98.7% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With nitric acid; silver nitrate In water | 98.5% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
106-96-7
propargyl bromide

-
-
101268-55-7
4,4-bis(ethoxycarbonyl)-1-hepten-6-yne

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran; hexane; toluene at -78℃; for 1h; | 98% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium ethanolate In diethyl ether; ethanol for 0.166667h; Cooling with ice; Inert atmosphere; Stage #2: propargyl bromide In diethyl ether; ethanol at 20℃; for 1h; Cooling with ice; Inert atmosphere; | 92% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.166667h; Inert atmosphere; Stage #2: propargyl bromide In N,N-dimethyl-formamide at 0 - 25℃; for 1h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium borohydride; zinc(II) iodide; cobalt(II) bromide-[1,2-bis(diphenylphosphino)ethane] In dichloromethane at 40℃; for 20h; | 98% |
| With cobalt(II) bromide-[1,2-bis(diphenylphosphino)ethane]; zinc(II) iodide; zinc In dichloromethane for 20h; | 92% |
| With tetrabutylammonium borohydride; zinc(II) iodide; cobalt(II) bromide-[1,2-bis(diphenylphosphino)ethane] In dichloromethane at 40℃; for 16h; | 81% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
821-10-3
1,4-Dichloro-2-butyne

-
-
1193378-70-9
tetraethyl dodeca-1,11-dien-6-yne-4,4,9,9-tetracarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; Stage #2: 1,4-Dichloro-2-butyne In tetrahydrofuran; N,N-dimethyl-formamide at 0 - 20℃; | 98% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
7022-45-9
2-(methylsulfanyl)benzaldehyde

-
-
1362860-33-0
1,3-diethyl 2-(4-(2-(methylthio)phenyl)-4-oxobutyl)malonate

| Conditions | Yield |
|---|---|
| With C21H44N2P2Rh*C32H12BF24 In acetone at 55℃; for 3h; Inert atmosphere; | 98% |
| With [Rh(η6-C6H5F)(bis(dicyclohexylphosphino)methane)][B(C6H3-3,5-(CF3)2)4] In 1,2-dichloro-ethane at 80℃; for 2h; Inert atmosphere; |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 65℃; for 0.166667h; Sealed tube; Stage #2: (Z)-3-fluoro-3-phenylallyl 2,2,2-trifluoroacetate With C51H54IrNO4P(1+)*BF4(1-) In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; enantioselective reaction; | 98% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
74-88-4
methyl iodide

-
-
53651-72-2
diethyl allylmethylmalonate

| Conditions | Yield |
|---|---|
| 97% | |
| With sodium methylate | |
| With sodium ethanolate |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
193806-54-1
2-Allyl-2-ethoxycarbonyl-succinic acid 4-tert-butyl ester 1-ethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride | 96% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.5h; Stage #2: bromoacetic acid tert-butyl ester In tetrahydrofuran; mineral oil at 20℃; for 16h; |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
106-93-4
ethylene dibromide

-
-
174619-42-2
diethyl (+/-)-(2-bromoethyl)(2-propenyl)propanedioate

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: ethylene dibromide In tetrahydrofuran at 20℃; for 15h; | 96% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 1.5h; Inert atmosphere; Stage #2: ethylene dibromide In tetrahydrofuran; mineral oil at 20℃; for 15.5h; Inert atmosphere; | 96% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: ethylene dibromide In tetrahydrofuran at 20℃; for 15h; | 81% |

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran Stage #2: C39H53NO3*C24H32O8*F6P(1-)*H(1+) With 1,3-bis-(diphenylphosphino)propane; palladium diacetate In tetrahydrofuran at 20℃; for 20h; Tsuji-Trost allylation; | 96% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
85850-08-4
(Z)-1-bromo-3-methylpent-2-en-4-yne

-
-
1187458-52-1
2-allyl-2-((Z)-3-methyl-pent-2-en-4-ynyl)-malonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran Inert atmosphere; Stage #2: (Z)-1-bromo-3-methylpent-2-en-4-yne In tetrahydrofuran Inert atmosphere; | 96% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
42201-43-4
2-allyl-1,3-propanediol

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 4.5h; Stage #2: With water; sodium hydroxide In tetrahydrofuran at 0 - 20℃; for 0.166667h; | 95% |
| With lithium aluminium tetrahydride In diethyl ether for 1h; Ambient temperature; | 94% |
| With lithium aluminium tetrahydride In diethyl ether 1.) 1 h, 0 deg C, 2.) 4 h, room temperature; | 86% |

| Conditions | Yield |
|---|---|
| With triethylsilane; t-Bu-P4 In hexane; N,N-dimethyl-formamide at 80℃; for 2h; | 95% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
50-01-1
guanidine hydrochloride

-
-
97570-29-1
5-allyl-2-aminopyrimidine-4,6-diol

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester; guanidine hydrochloride With sodium In ethanol at 20℃; for 4h; Inert atmosphere; Stage #2: In ethanol for 1h; Inert atmosphere; Reflux; | 95% |
| With sodium In ethanol Inert atmosphere; | 95% |
| With sodium methylate In methanol at 0℃; Reflux; | 62.8% |
| With sodium carbonate In water at 60℃; for 0.5h; Reagent/catalyst; Sonication; | 61% |
| Stage #1: guanidine hydrochloride With sodium ethanolate In ethanol at 0℃; for 0.166667h; Stage #2: (2-propenyl)propanedioic acid diethyl ester In ethanol at 0 - 20℃; for 65h; Stage #3: With hydrogenchloride In ethanol; water | 51% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
20629-39-4, 20629-62-3, 79629-38-2
1,2-dibromo-2-butene

-
-
1254219-12-9
diethyl 2-allyl-2-((Z)-2-bromo-2-butenyl)malonate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In tetrahydrofuran at 20℃; Inert atmosphere; | 95% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
21003-54-3, 23970-90-3, 96725-21-2
(E)-(2,3-dibromoprop-1-enyl)benzene

-
-
1254219-14-1
diethyl 2-allyl-2-((E)-3-phenyl-2-bromo-2-propenyl)malonate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In tetrahydrofuran at 20℃; Inert atmosphere; | 95% |

-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
1304145-47-8
diethyl 2-allyl-2-(penta-2,3-dien-1-yl)malonate

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: penta-2,3-dien-1-yl methanesulfonate In tetrahydrofuran; mineral oil at 21 - 25℃; Inert atmosphere; | 95% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Stage #2: penta-2,3-dien-1-yl methanesulfonate In tetrahydrofuran at 0 - 55℃; for 50.0833h; | 40% |

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; hydrogen In ethanol at 20℃; for 4h; | 94% |
| With hydrogen; polymeric rhodium In tetrahydrofuran; ethanol under 760 Torr; for 12h; |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
18495-27-7
1-bromooct-2-yne

-
-
350248-68-9
(2-octynyl)-(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; | 94% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 0 - 20℃; Stage #2: 1-bromooct-2-yne In tetrahydrofuran at 20℃; for 16h; Further stages.; | 81% |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

-
-
1794-48-5
1-bromo-3-phenylprop-2-yne

-
-
143633-91-4
diethyl 2-allyl-2-(3-phenylprop-2-yn-1-yl)malonate

| Conditions | Yield |
|---|---|
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 20℃; Stage #2: 1-bromo-3-phenylprop-2-yne In tetrahydrofuran at 20℃; for 12h; Further stages.; | 94% |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1.5h; Inert atmosphere; Stage #2: 1-bromo-3-phenylprop-2-yne In tetrahydrofuran; mineral oil at 0 - 20℃; for 24h; Inert atmosphere; | 79% |
| With sodium hydride Inert atmosphere; | |
| Stage #1: (2-propenyl)propanedioic acid diethyl ester With sodium hydride In tetrahydrofuran at 0 - 20℃; for 1.5h; Stage #2: 1-bromo-3-phenylprop-2-yne In tetrahydrofuran at 0 - 20℃; |
-
-
2049-80-1
(2-propenyl)propanedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With N,O-bis-(trimethylsilyl)-acetamide; {(η4-1,5-cyclooctadiene)Pd(η3-allyl)}BF4; 1,1′-binaphthalene-2,2′-diylbis[bis(4-methylphenyl)phosphine] In 1,4-dioxane at 60℃; for 24h; Inert atmosphere; enantioselective reaction; | 94% |
Diethyl allylmalonate Chemical Properties
Molecular structure of Propanedioic acid, 2-(2-propen-1-yl)-, 1,3-diethyl ester (CAS NO.2049-80-1):
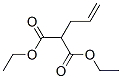
IUPAC Name: Diethyl 2-prop-2-enylpropanedioate
Molecular Weight: 200.23164 [g/mol]
Molecular Formula: C10H16O4
Density: 1.021 g/cm3
Boiling Point: 222.5 °C at 760 mmHg
Flash Point: 92.8 °C
Index of Refraction: 1.439
Molar Refractivity: 51.61 cm3
Molar Volume: 196 cm3
Surface Tension: 31.8 dyne/cm
Enthalpy of Vaporization: 45.9 kJ/mol
Vapour Pressure: 0.101 mmHg at 25 °C
XLogP3: 1.9
H-Bond Acceptor :4
Rotatable Bond Count: 8
Exact Mass: 200.104859
MonoIsotopic Mass: 200.104859
Topological Polar Surface Area: 52.6
Heavy Atom Count: 14
Canonical SMILES: CCOC(=O)C(CC=C)C(=O)OCC
InChI: InChI=1S/C10H16O4/c1-4-7-8(9(11)13-5-2)10(12)14-6-3/h4,8H,1,5-7H2,2-3H3
InChIKey: GDWAYKGILJJNBB-UHFFFAOYSA-N
EINECS: 218-072-9
Product Categories: Pharmaceutical Intermediates; Aromatic Esters; TheMalonateRamificationProducts
Diethyl allylmalonate Uses
Propanedioic acid, 2-(2-propen-1-yl)-, 1,3-diethyl ester (CAS NO.2049-80-1) is used as raw materials for pharmaceutical and pesticide intermediates.
Diethyl allylmalonate Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 3
HS Code: 29171990
Diethyl allylmalonate Specification
Propanedioic acid, 2-(2-propen-1-yl)-, 1,3-diethyl ester (CAS NO.2049-80-1) is also named as AI3-11069 ; Diethyl 2-(2-propenyl)-1,3-propanedioate ; Diethyl 2-(prop-2-enyl)malonate ; Diethyl 2-allylmalonate ; Diethyl allylmalonate ; Diethyl prop-2-enylpropanedioate ; Ethyl allylmalonate ; Malonic acid, allyl-, diethyl ester ; NSC 67393 ; NSC 8777 ; Propanedioic acid, 2-propenyl-, diethyl ester . Propanedioic acid, 2-(2-propen-1-yl)-, 1,3-diethyl ester (CAS NO.2049-80-1) is clear colorless to slightly yellow liquid.
Related Products
- Diethyl 1,1-cyclopropanedicarboxylate
- Diethyl 1,3-acetonedicarboxylate
- Diethyl 1-benzylpyrrolidine-2,5-dicarboxylate
- Diethyl 1H-imidazole-4,5-dicarboxylate
- Diethyl 2-(1H-indol-3-ylmethyl)propanedioate
- Diethyl 2-(2,2-diethoxyethyl)propanedioate
- Diethyl 2-(2-chloroethyl)propanedioate
- Diethyl 2-(2-cyanoethyl)malonate
- Diethyl 2-(2-oxocyclohexyl)malonate
- Diethyl 2-(4-(benzyloxy)benzylidene)malonate
- 2049-94-7
- 2049-95-8
- 2049-96-9
- 2050-01-3
- 2050-08-0
- 2050-20-6
- 2050-22-8
- 2050-23-9
- 2050-25-1
- 20503-40-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View